
Neurosurgery Research
Neurosurgery is at the frontier of medical science working to discover the secrets of the brain to better understand and treat patients. Learn more about the Department of Neurosurgery.
About
One of the most concerning experiences is hearing that you or a loved one is being referred to a brain surgeon.
Brain or spine diseases such as cancer, epilepsy or chronic pain can be physically and mentally taxing on patients and their loved ones during all stages of disease progression. The home health care needs sometimes associated with brain conditions requiring surgery can add considerable financial and social burdens.
The Carilion Clinic Neurosurgery team is committed to providing the best care possible—support, education and treatment—to optimize every patient's quality of life. We believe that this philosophy includes the pursuit of new ideas and innovations to better investigate and bring new technologies to help heal our community.
When asked why they become neurosurgeons, many doctors will mention the beauty of the "black box" called the brain. The brain is not only a biological computer, running the human body; it is also responsible for our personalities, memories and core selves. Each brain and thus each person is unique. Much remains unknown about the brain and how all the individual pieces work together to create the wonderful being that is a human person.
Our neurosurgical team is dedicated to exploring the brain and its secrets; finding new ways to understand the healthy brain and disease; and helping to create the newest, advanced treatments for our patients.
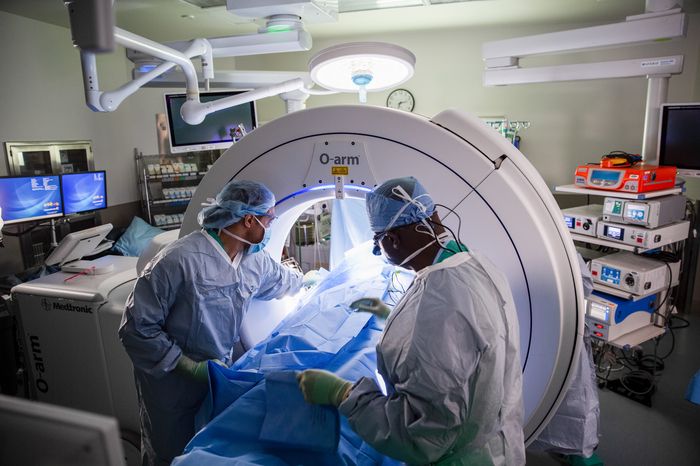
"At Virginia Tech Carilion Neurosurgery, we believe that clinical, translational and basic science research are fundamental to the development of the field of neurosurgery. To this end, we focus many resources on developing our faculty and trainees as research thought leaders. To accomplish this, we participate in many investigator sponsored studies as well as clinical trials, and regularly host visiting faculty to foster the development and interchange of new ideas."
Mark Witcher, MD, PhD
Resident Program Director
Common Conditions
Neurosurgeons treat a variety of complex conditions relating to the brain, nerves and nervous system, as well as its supporting structure, such as brain and spine. Some of the more common conditions can be broken down into the following categories:
- Brain or cranial swelling
- Cancers
- Carpal tunnel syndrome
- Congenital conditions
- Movement disorders (e.g., Parkinson's disease, epilepsy, etc.)
- Nervous system infections
- Pain disorders
- Spinal disorders (including degenerative disc/vertebral body disorders)
- Traumatic injuries
- Vascular disorders (e.g., stroke, hemorrhages, hypertension, aneurysms)

Current Research
Neurosurgery research is as varied and far-reaching as the conditions neurosurgeons treat, and our portfolio of ongoing research is continually growing. Highlighted below are some of the prominent areas of our current neurosurgery research in the areas of:
- Cancer
- Imaging
- Innovation
- Movement Disorders
- Pain
- Traumatic Injury
- Vascular
Cancer
Patient-Derived Tissue-Engineered Systems With Physiological Flow to Study Glioblastoma
PI: Mark Witcher, MD, PhD
Collaboration with Jenny Munson, PhD, Virginia Tech
Cancers in the brain are difficult to treat. Efforts to identify new treatments for glioblastoma (GBM), the most common malignant and deadly brain cancer, have stagnated since the creation of the highly successful chemotherapy drug, temozolomide, in 2005 that largely improved survival and treatment. Since then, little has been accomplished to increase survival in patients with GBM.
Most studies of GBM in research labs have focused on the cells of the cancerous tissue themselves, rather than the location and characteristics of the environment of the tumor. To improve our experiments and the relevance of our results, we have invented a new study system—the tumor microenvironment (TME)—where we make our samples with tumor cells in conjunction with other normal brain cells to recreate the way the tumor cells and normal brain cells interact.
In this research, we will collect the brain tumor tissues and cerebrospinal fluid (CSF) that would both otherwise be discarded after surgery. The amount of CSF collected will vary depending on the procedure of the voluntary participant. We will generate TME samples with cells isolated from these collected patient samples, then treat the samples in the laboratory with temozolomide and radiation, mimicking the standard treatment that patients receive for GBM. We will measure the response of the TME samples by seeing how much tumor cells travel and go through cell death, then compare the treatment-induced changes in the experimental samples with the course and outcome of the actual patient’s GBM disease from clinical treatment after surgery.
By comparing the responses of the TME samples to the patient’s outcome, we will determine if our TME sample system will be able to predict the patient's response to treatment.
At the conclusion of this study, we will have the data and information needed to propose a clinical trial in which we would set up our patient TME samples and treat with standard post-surgery treatment for GBM (chemotherapy and radiation) and provide the results to the treating physicians so that they can consider altering the patient’s treatment with hopes of improving patient outcomes and course of disease.
Imaging
PET-based Imaging of High-Grade Gliomas using Gallium-labeled Prostate-Specific Membrane Antigen (PSMA)
PI: Mark Witcher, MD, PhD
Humans with high-grade gliomas—tumors of the brain or spinal cord—have a poor prognosis, with an average survival time of just 12-18 months for patients who undergo standard tumor removal and traditional radiation and/or chemotherapy.
Currently, surgery and chemoradiotherapy serve as standard treatment for this condition, yet these can be complicated by the tumor location, growth rate and recurrence. A recurrent tumor can often be very difficult to distinguish using conventional gadolinium-based MRI imaging, given its similarity in appearance to normal radiation-induced changes.
Despite these shortcomings, MRI serves as the dominant imaging technique, which likely doesn’t reveal the entirety of a tumor’s profile (de novo or recurrent) due to limitations inherent to gadolinium contrast, the current contrast dyes used to improve MRI image quality. Other imaging modalities, including PET, could play a significant role in the workup and treatment of these tumors.
To this end, we must identify PET-based markers for use in imaging of high-grade gliomas. Gallium-labeled prostate-specific membrane antigen (68Ga-PSMA) has demonstrated success as an effective target often associated with high-grade gliomas and will be evaluated in this study to diagnose these tumors.

Innovation
Local, Biodegradable, Sustained-Release Drug-Delivery System for Prevention of Chronic Subdural Hematoma (cSDH) Recurrence
PIs: Kristine Ravina, MD, and Eric Marvin, DO
Collaboration with Abby Whittington, PhD, Virginia Tech
Chronic subdural hematoma (cSDH), or long-term brain bleeding, is one of the most common neurosurgical diseases. It involves blood buildup in the space below the skull, typically due to initial connective vein tear that has worsened over a period of at least 3 weeks. This process usually involves breakdown of the blood products, membrane formation and development of long-term inflammatory changes.
The incidence of cSDH dramatically increases in those aged 70 years or older. Recurrent cSDH is reported in 10–25% of patients after surgical evacuation and can lead to devastating long-term consequences. Few studies to date have reported effectiveness of wholistic anti-inflammatory treatment in cSDH, such as corticosteroids (e.g., dexamethasone and prednisone) that, while demonstrating promising decrease in recurrence rates in some studies, have without fail been followed by systemic (whole body) side-effects and increased death rates. To date, there have been no reports of potential local (site of bleed) drug-delivery system use in cSDH patients to reduce recurrence rates and side effects from systemic use.
The idea of incorporating a commonly used antibiotic and an anti-inflammatory corticosteroid in a local drug delivery system is attractive given their individual robust effects on the core processes involved in cSDH. Additionally, the use of currently FDA approved medications and drug delivery systems/concepts has the potential in streamlining in-human clinical trials and rapid commercialization of these products for wide-spread use. Given cSDH are often requiring intense surgical evacuation via burr holes or a craniotomy, it allows for a unique window of local drug delivery system application thus avoiding an additional surgery to deliver the new drug to the site of cSDH.
____________
Retractor Project
PI: Adeolu Olasunkanmi, MD
____________
Novel Dural Sealant
PIs: Adeolu Olasunkanmi, MD
Collaboration with John Matson, PhD, and Michael Schulz, PhD, Virginia Tech
Dural tears-ruptures in the membrane (dura mater) that surrounds the brain and spinal cord-are one of the most challenging complications during neurosurgery, occurring during lumbar spinal surgery, for example, in 3.1-17.4% of cases. Inevitably, dural tears result in cerebrospinal fluid (CSF) leakage, increasing risk of infections and complications. To mitigate these complications, surgical materials have been developed to repair dural tears, including sutures, fibrin-adhesive sealants, and gel foam; however, a fully effective approach to mitigating CSF leaks remains elusive. Commercially available sealants, covalent bonds form between the amines in the underlying dural tissue and the NHS-esters in the formulation, producing an adhesive hydrogel. PEG-based sealants are hydrophilic and consequently swell in aqueous or humid environments, resulting in water uptake ranging from 87-558%. This swelling limits certain applications of these products. For example, commercially-available dural sealants are contraindicated for use in confined bony structures where nerves and spinal cord are present because hydrogel swelling may produce neural compression. PEG-based sealants, and other polyether-based materials, are also not fully biodegradable.
The goal of this work is to develop novel, improved materials for sealing dural tears. Most commercial dural sealants are based on legacy materials that were never specifically developed for neurosurgical applications. Consequently, current dural sealants are inherently limited by the underlying molecular structure of the materials themselves. As working with collaborator polymer synthesis group at VT, they will tune the molecular structure of the sealant material to balance dural adhesion, cohesive (internal) strength, CSF resistance, and ease of application.
____________
Flow Sensor for Ventriculoperitoneal (VP) Shunt Project
PI: Mark Witcher, MD, PhD
Having a buildup of cerebrospinal fluid (CSF) can cause certain brain conditions that require drainage of the fluid to prevent more serious conditions. Ventriculoperitoneal (VP) Shunts are simple systems that drain excess CSF once implanted. The CSF is released into tissue in the abdomen which can manage CSF and effectively dispose of it. This shunt system is implanted underneath the skin and flow is regulated by the valve. Current editions of this technology are purely based on buildup of CSF, as the catheters are passive components, and the valve is either physically self-managed through changes in pressure or externally managed using a magnetic system. This shunt system can be prone to issues arising from infection and blockages within the shunt line that aren’t detected by the passive system. Typically, patients will then present in the emergency department extremely ill and requiring a second procedure to replace the shunt system. We are interested in adding a flow rate monitor and a wireless information relay to monitor the shunt’s effectiveness and prevent patients from becoming ill or having poor outcomes from a failing device.
In this study, a device will be placed in the external drain line (catheter) of patients that have had a shunt placed in their head. This device will be monitored, and data recorded via hooking up the electrodes to a device called the Arduino (a small data recording device). Multiple data points will be collected about the new medical device performance, stability, flow capability, durability, etc. as the design is tweaked for the best possible version of a new medical device to be ultimately created and used in the field. Additionally, the CSF will be tested by collection via the catheter port and testing for potential ion presence to determine its impact on the device, as well as chart review for labs and demographics that may impact the new device.

Movement Disorder
Neurochemical and Neurophysiological Assessment During Surgery: Parkinson’s, Dystonia and Essential Tremor
PI: Mark Witcher, MD, PhD
Collaboration with Read Montague, PhD, Virginia Tech
One important issue for understanding diseases and disorders that involve dopaminergic systems, any system or disease that involves the dopamine neurotransmitter (like brain degenerative disorders like Parkinson’s), is to establish an undeniable link between the body’s internal events involved in reward processing and the ? (maybe not understanding correctly – Andy) negative disordered outcomes involved in disease that these events lead to. There is a large and growing body of knowledge concerning the molecular and cellular processes involved in reward pathways and the way in which they are influenced by drugs typically seen in abuse and neurological disorders. Despite the rapid progress in experimental methods for studying dopaminergic systems, there is still a large gap in our knowledge concerning the type of information processing mechanisms that these systems carry out. The broad objective of this proposal is to establish one such link by providing a computational working understanding of the kinds of information constructed and broadcasted by midbrain dopamine systems and the influence of these signals on their following neural targets.
This study will provide functional data concerning brain function and structure using active and passive reward-oriented tasks to uncover changes in the brain’s chemical activity while undergoing deep brain stimulator implantation surgery where electrodes are placed deep in the brain and stimulate the brain to basically rid itself of abnormal activity. Since much work in this system has been done in the intact human brain, the goal here is to understand the parts of the brain associated with higher order cognitive processes such as reward processing and decision-making, at both functional and structural levels. Some subjects may have the opportunity to participate in an fMRI study prior to this study using same or similar reward-oriented tasks. No prior fMRI study participation is required for the current proposed study. If subjects in the current study have also participated in a prior fMRI study, the results will be compared.
Additional Information
Studies on mesencephalic dopamine systems in behaving animals suggest that activity changes in dopamine neurons of the ventral tegmental area and substantia nigra represent computationally important signals, that is, dopamine fluctuations represent errors in predictions of the time and amount of future rewarding events (see Schultz et al. 1997 for review). These errors can be transmitted via changes in the spiking activity of the neurons and resultant fluctuations in dopamine (Montague and Sejnowski, 1994; Montague et al., 1996; Schultz et al., 1997; Montague et al., 2004; Bayer and Glimcher, 2004). Further changes in dopaminergic target structures have been found in humans using fMRI. Blood oxygen level dependent (BOLD) responses measuring reward prediction error signals can be dissociated in the striatum according to whether an action is required for the acquisition of the reward. During active and passive tasks, the reward prediction error is evident in the ventral striatum (McClure et al., 2003, O'Doherty et al., 2003). In active tasks, however, a strong response in the dorsal striatum also appears (O'Doherty et al., 2003). Computational models also suggest that a decrease in dopamine in the system may result in delay times for reward decision making (Montague et al., 1996). It is therefore reasonable to suspect that the above computational interpretation of dopaminergic activity will yield novel insights into those aspects of reward processing and decision making over which dopamine has a strong influence.
Diseases and disorders that disrupt the dopaminergic system provide an opportunity to investigate this system in varying states of dysfunction. Parkinson's disease (PD) is characterized by progressive loss of nigro-striatal dopaminergic neurons (Mayeux, 2003). Dopamine depletion in the striatum results in motor deficits, such as akinesias, rigidity, and tremor. Dopamine depletion can also cause other nonmotor features such as depression, anxiety, and cognitive impairment. Dopamine replacement treatment is common in Parkinson’s disease and reduces the motor deficits. Long-term treatment with L-dopa, the precursor to dopamine synthesis, can induce other motor deficits and motor fluctuations (Wichmann and DeLong, 2006).
Another form of treatment for certain PD patients is deep brain stimulation (DBS) to modify cortico-basal ganglia circuits. Stimulation electrodes are inserted into specific brain regions (globus pallidus, thalamus, subthalamic nucleus, or pedunculopontine nucleus) and high-frequency stimulation is delivered via an externally programmable pulse generator. This stimulation results in immediate effects on motor deficits and reduces the dose of medication needed to target other residual disease symptoms (Kringelbach et al.,2007). Indeed, the success of DBS for Parkinson's disease has led to its use in the treatment of other neurological and neuropsychiatric diseases, such as obsessive-compulsive disorder, depression, multiple sclerosis, Tourette’s syndrome, and chronic pain.
In the instance of Parkinson's disease, the best candidates for DBS are patients with levo-dopa responsive PD that have no psychiatric symptoms (Wichmann and DeLong, 2006). In this instance, DBS electrodes are implanted bilaterally, one hemisphere at a time. During implantation of the electrode, the neural activity surrounding the electrode sites is recorded to determine the location of the electrode in the brain. Sites such as the thalamus, subthalamic nucleus, and substantia nigra have specific neural activity patterns. Microelectrode mapping of these areas in response to passive and active movements is necessary to determine the upper and lower boundaries of the subthalamic nucleus to ensure proper placement of the electrode. After the electrode is positioned in the subthalamic nucleus, a brief neurological exam is performed to determine the proper generator settings for reduction in tremor and rigidity without concomitant alterations in motor and cognitive function. This procedure is repeated in the opposite hemisphere.
- Bayer HM, Glimcher PW. (2005) Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47:129-41.
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. (2007) Translational principles of deep brain stimulation. Nat Rev Neurosci 8: 623-35.
- Mayeux R. (2003) Epidemiology of neurodegeneration. Annu Rev Neurosci 26: 81-104.
- McClure SM, Berns GS, Montague PR. (2003) Temporal prediction errors in a passive learning task activate human striatum. Neuron 38: 339-46.
- Montague PR, Sejnowski TJ. (1994) The predictive brain: temporal coincidence and temporal order in synaptic learning mechanisms. Learn Mem 1: 1-33.
- Montague PR, Dayan P, Sejnowski TJ. (1996) A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 16: 1936-47.
- Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, Kilpatrick MR, Wightman RM. (2004) Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci 24: 1754-9.
- Schultz W, Dayan P, Montague PR. (1997) A neural substrate of prediction and reward. Science 275: 1593-9.
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. (2003) Temporal difference models and reward- related learning in the human brain. Neuron 38: 329-37.
- Wichmann T, Delong MR. (2006) Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52: 197-204
____________
Neural Dynamics of Epilepsy Patients
PI: Mark Witcher, MD, PhD
Collaboration with Sujith Vijayan, PhD, Virginia Tech
This project will generate large datasets using intracranial electrodes, electrodes placed inside the skull usually at potential sites of seizure origin, implanted in patients with uncontrolled epilepsy to study the neural dynamics of the brain and what role sleep may have in motor learning, memory ordering and storage, responses to stimuli, and mental imagery. We additionally will investigate the neural activity in different behavioral states and sleep stages to better our understanding of the sleeping brains communication and organization of information for future use.
____________
Electrochemical Measurements During Epilepsy Monitoring Procedures
PI: Mark Witcher, MD, PhD
Collaboration with Read Montague, PhD, Virginia Tech
Dysfunction in dopamine, serotonin, or norepinephrine associated brain signaling can result in a range of psychiatric conditions including depression, anxiety, chronic pain, addiction disorders, and problems with attention (attention deficit disorder) and arousal (narcolepsy). Despite the clear importance of these types of signaling systems and pathways, we know practically nothing about how these systems act in real-time (at sub-second timescales) in the human brain. The purpose of this research study is to gain basic knowledge about how the human brain works. Specifically, the goal of this study is to measure the levels of dopamine, serotonin, and norepinephrine throughout the human brain and how these levels respond to reward in both passive and active situations. Subjects will consist of patients undergoing phase-II epilepsy monitoring. Functional MRI recordings will be carried out separately from the subject’s clinical stay if they consent to the optional portion of the study. A separate set of study tasks will be performed during fMRI scanning. Neurotransmitter recording will be carried out using clinical electrodes already implanted for epilepsy monitoring. Using refined techniques, we will be able to use recordings obtained from these electrodes to measure at sub-second resolution concentrations of dopamine, serotonin, and norepinephrine all at the same time. Understanding the behavior of these neurotransmitters will have far reaching implications in the treatment of many psychiatric and neurological conditions.
Additional Information
This temporal resolution has proven to be important in basic research in rodent model organisms, where it has been shown that each of these neurotransmitters’ extracellular concentration changes within 100s of milliseconds of interacting with relevant stimuli while navigating moment-to-moment changes in the environment. Measurements with this kind of precision are necessary to be able to investigate how rapid changes in each of these signals modulate brain function, mood, and behavior in humans.
Currently, there is no technology available that will permit simultaneous sub-second measurements of dopamine, serotonin, and norepinephrine in the human brain. However, the PI of this proposal (Montague) has recently developed a novel approach that enables real-time measurements of dopamine and serotonin using carbon fiber micro-sensors and a machine learning based approach to fast scan cyclic voltammetry (Kishida et al., 2011; Kishida et al., 2016; Moran et al., 2018). Further, preliminary evidence from the investigators’ lab suggests that this technology may be extended to simultaneously measure dopamine, serotonin, and norepinephrine in the human brain. Here, we propose to deploy a new recording protocol in combination with an FDA approved micro-sensor assembly (for epilepsy mapping) that combined will enable (for the first time ever) simultaneous measurements dopamine, serotonin, and norepinephrine micro-fluctuations with sub-second temporal resolution in the human brain. If successful, the proposed work could provide a significant technological advance for neuroscience research in human brain function and behavior, with potential translational impact in areas including in neurosurgery, neurology, and psychiatry.
Clinical context of proposed research: Patients with treatment (medication) resistant epilepsy can become candidates for surgical ablation of the epileptic foci. To determine which region of the brain is the source of the seizures patient undergo phase-I and if necessary phase-II epilepsy monitoring. Phase-I requires non-invasive electroencephalographic recordings using electrodes placed on the patient’s scalp. If these recordings are inconclusive, patient become candidates for intracranial depth electrode mapping, which requires brain surgery to place the depth electrodes (e.g., see Fig. 1) as shown in Fig. 2. An alternative depth electrode that is used for clinical purposes is AdTech’s “All-in-one Macro-Micro” electrode, where the micro contacts are radially spaced on the body of the depth electrode. These electrodes are FDA approved for standard-of-care use side-by-side with clinical research recordings. These electrodes and this procedure are the platform for the recordings we propose here. Importantly, we will only perform research recordings from sites that will necessarily be damaged by the clinical procedures, and due to the unknown epileptic foci will later be deemed either healthy or epileptic tissue.
Background to electrochemical measurement approach. The critical measurement approach that enables simultaneous electrochemical detection of dopamine, serotonin, and norepinephrine is an electrochemical method called “fast scan cyclic voltammetry”. This approach has been utilized in rodents and rodent brain tissue for over 25 years. Briefly, a voltage is applied to a carbon fiber microelectrode. If this voltage is greater than or equal to the oxidation potential of a chemical species, then an electrochemical oxidation reaction takes place and the transfer of electrons (to the carbon fiber) is measured as a change in current. By quickly scanning over a range of applied potentials (e.g., -0.6V to +1.4V and back to -0.6V at a scan rate of 400V/s), a range of electrochemical currents can be detected. We have developed a machine learning based algorithm that allows us to infer the chemical species’ identity and concentration from this induced electrochemical spectrum. Tests like those shown in Figure 3 demonstrate that we can continuously monitor dopamine, serotonin, and norepinephrine micro-fluctuations with 100ms temporal resolution, which is orders of magnitude faster and with direct chemical specificity better that existing non-invasive measurement modalities like fMRI and PET and invasive approaches like microdialysis.
The macro-micro electrodes used will be identical to those utilized in previous IRB-approved procedures for epilepsy monitoring.
The clinical importance of investigating the action of the neurotransmitters dopamine, serotonin, and norepinephrine is perhaps best highlighted by the pharmaceuticals used to treat major psychiatric conditions like depression, anxiety disorders, chronic pain, attention deficit disorders, and nicotine addiction. Selective serotonin reuptake inhibitors (SSRIs) are used to treat depression and anxiety; Norepinephrine and Serotonin reuptake inhibitors (NSRIs) are used to treat depression and chronic pain; Norepinephrine and dopamine reuptake inhibitors are used to treat depression, attention deficit disorders, and have been used as an aid to smoking cessation; and Norepinephrine reuptake inhibitors (NRIs) have been used to treat depression, narcolepsy, attention deficit hyperactivity disorder, as an aid to weight loss, and anxiety disorders characterized by low arousal. Furthermore, abused substances (e.g., cocaine, nicotine, alcohol, and opiates) are known to alter the subtle balance between neurotransmitter release and reuptake in model organisms.
From a basic science perspective, we believe that dopamine is critical for reward processing and motivated behavior, serotonin for processing aversive stimuli and mood regulation, and norepinephrine for regulating states of arousal and attention. These neurotransmitters are released from neurons located in the brain stem (serotonin and norepinephrine) and midbrain (dopamine) whose axon terminals distribute and broadcast these signals throughout the brain including targets throughout the cortex (dopamine, serotonin, and norepinephrine), basal ganglia (dopamine and serotonin), hippocampus (dopamine and serotonin), and amygdala (dopamine, serotonin, and norepinephrine). While we know that these systems are critical, most of what we know comes from model organism research at timescales too slow to understand how rapid, real-time fluctuations in these signals contribute healthy human cognition, decision-making, and behavior. Additionally, very little is known about the neurophysiological processes involved in mindfulness. Recently, studies have established that subjects with experience in mindfulness shows attenuation of reward-related responses in ventral striatum (Kirk et al., 2019, 2015). In line with this finding is previous work demonstrating that changes in mental states, such as employing emotion regulation strategies, may regulate reward expectation (Delgado et al., 2008; Gu et al., 2014). This proposal allows us to examine to which degree training in mindfulness will lead to changes both behaviorally and in the underlying neurochemistry.
We also have a very limited understanding of how dopamine, serotonin, and norepinephrine systems interact. In any given brain region, it may be expected that there are one, two, or all three of these neurotransmitter systems contributing to the local neural information processing. In the human brain (and non-human primate brain) we know little about how the density of release sites or the dynamics of release change with psychiatric conditions or the medications used to treat them. This lack of knowledge does not stem from a lack of interest in the neuroscience, neurology, psychiatry, or neurosurgery disciplines; rather, the necessary technology and research paradigm has not been available. This proposal seeks to take the first steps in developing novel hardware (micro-sensor assembly) and pair it with the PI’s machine learning based approach to fast scan cyclic voltammetry to simultaneously measure continuous sub-second microfluctuations of dopamine, serotonin, and norepinephrine in the human brain.
Simultaneous sub-second measurements of dopamine, serotonin, and norepinephrine in the human brain would allow investigators to monitor how these three neurotransmitters fluctuate in real-time. Such technology could potentially be used to develop real-time biomarkers of dynamic brain activity in disease specific brain areas, which may be used diagnostically or prognostically in psychiatry (e.g., depression or OCD) and neurology (e.g., Parkinson’s disease or epilepsy) and neurosurgery (e.g., deep brain stimulation electrode placement or lesion/tumor resection boundaries). Further, such measurements could be used to assess exactly how drugs (clinical treatments or abused substances) alter the function of these neuromodulatory systems in real-time in the human brain. Finally, such technology could provide a breakthrough in intracranial neuroscience research into the basic neural mechanisms underlying decision-making processes that may generally be affected in humans prone to poor health-related behaviors.
The questionnaires and assessments proposed will provide insights into disorders (and anxiety, depression) and psychological status that we hope to understand in relation to the neurochemistry measures. They will also provide baseline information that may be used to characterize and group the population to further refine our understanding of the neural responses.
References
- http://adtechmedical.com/depth-electrodes
- http://www.epilepsy.com/
- *Moran, R.J., *Kishida, K.T., *Lohrenz, T., Saez, I.G., Laxton, A.W., Witcher, M., Tatter, S., Elllis, T.L., Phillips, P.E.M., Dayan, P., and Montague, P.R. (2018) The protective action encoding of serotonin transients in the human brain. Neuropsychopharamcology, 43: 1425-1435. doi: 10.1038/npp.2017.304
- Kishida, K.T., Saez, I.G., Lohrenz, T., Witcher, M., Laxton, A., Tatter, S., White, J.P., Elllis, T.L., Phillips, P.E.M., and Montague, P.R., (2016). Sub-second dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. Proceedings of the National Academy of Sciences, U.S.A. 113(1): 200-205. doi:10.1073/pnas.1513619112. PMID: 26598677.
- *Kishida, K.T., *Sandberg, S.S., Lohrenz, T., Comair, Y.G., Saez, I.G., Phillips, P.E.M., and Montague, P.R. (2011). Sub-Second Dopamine Detection in Human Striatum. PLoS ONE. 6(8): e23291. PMID: 21829726.
- Gu X, Kirk U, Lohrenz TM, Montague PR. Cognitive strategies regulate fictive,
but not reward prediction error signals in a sequential investment task. Hum
Brain Mapp. 2014 Aug;35(8):3738-49. - Kirk U, Montague PR. Mindfulness meditation modulates reward prediction errors
in a passive conditioning task. Front Psychol. 2015 Feb 12;6:90. - Kirk U, Pagnoni G, Hétu S, Montague R. Short-term mindfulness practice
attenuates reward prediction errors signals in the brain. Sci Rep. 2019 May
6;9(1):6964. - Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via
cognitive strategies. Nat Neurosci. 2008 Aug;11(8):880-1.
Pain
DePuy Synthes Spine Clinical Registry (CONDUIT™ Interbody Platform /EIT Cellular Titanium®)
PI: Gregory Howes, DO
Spinal fusion, basically re-joining spinal bones, is often a solution for patients experiencing chronic back or neck pain. Spinal fusion is a surgical procedure where two or more vertebrae are combined to form one continuous bone by removing damaged or deficient discs between the two vertebrae. The goal of spinal fusion surgery is to kickstart bone growth between two or more vertebrae using replacement synthetic or cadaver collected bone and a spacer in the intervertebral space. Once new bone forms, the vertebrae will be linked together, and there should be no further movement between the fused segments, which is what contributed to the patient’s pain. This study’s goal is to review patient’s experience in pain relief, device effectiveness, complications, and radiographic (imaging) findings of the CONDUIT™ Interbody Platform /EIT Cellular Titanium in a post market analysis being conducted at the behest of DePuy Synthes Spine.
For more information please see: Neurosurgery Research | Carilion Clinic
____________
Investigation of Low-Intensity Focused Ultrasound for the Control of Chronic Low Back Pain
PI: Mark Witcher, MD, PhD
Collaboration with Wynn Legon, PhD, Virginia Tech
Chronic pain is a major public health problem. An estimated 100 million Americans have experienced chronic pain leading to significant economic and social burden. The estimated annual cost of managing chronic pain in the United States is as high as $635 billion per year. Pharmacological treatments frequently require the use of addictive pain-killing opioids resulting in a major epidemic of abuse in the United States. New, non-addictive treatments for pain are needed. Neuromodulation, or the alteration of nerve activity with targeted electrical signals, with low intensity focused ultrasound (LIFU) may provide a non-pharmacological treatment. The enormous potential of LIFU stems from the ability to focus ultrasound through the intact skull to a very specific and precise target spot size anywhere in the brain. This makes it a powerful alternative to both invasive neurosurgical procedures and other noninvasive brain stimulation techniques. The anterior insula (AI) and the posterior insula (PI) are promising targets to treat chronic pain. Multiple lines of evidence support the involvement of the dorsal anterior insula in the intensity of the pain while the posterior insula is thought to signal the affective or emotional response from the pain to the brain which guides behavioral response to the pain. Shutting or slowing down either of these regions may serve to reduce the overall pain experience. Unfortunately, both areas lie deep to the cortical surface of the brain preventing access using conventional noninvasive techniques like transcranial magnetic stimulation (TMS) and transcranial electric stimulation (TES) that have poor accuracy and lack depth penetration. It is the purpose of this proposal to investigate the influence of neuromodulation with LIFU to either the AI or PI on distinct aspects of the pain experience (intensity and emotional response) in patients waiting to receive a dorsal column stimulator (DCS) for chronic pain, a standard of care treatment of chronic low back and radicular pain. It is the long-term goal of this project to advance LIFU as a human pain management tool to enhance the health and reduce suffering in those with chronic pain.
Additional Information
LIFU for spatially-targeted neuromodulation. LIFU is a novel neuromodulatory approach which uses mechanical energy to non-destructively and reversibly modulate neuronal activity with high spatial resolution and adjustable depth of focus 1–7. LIFU has been used safely and effectively for cortical and sub-cortical neuromodulation in mouse 8–12, rat 13–16, rabbit17, sheep18,19, pig20 and primate21-26. It is also an effective method of transient cortical and sub-cortical neuromodulation in humans1,2,27-35.
Human LIFU. We have extensive experience testing LIFU for cortical and sub-cortical neuromodulation in humans1,2,27,34,36-39. We precisely targeted the primary somatosensory cortex (S1) in humans and attenuated somatosensory evoked potentials (SEPs) generated in the posterior bank of the central sulcus 1. Additionally, LIFU altered tactile sensitivity as compared to sham stimulation. Movement of the transducer by one centimeter removed the effect - validating the spatial specificity of the neuromodulation. Subsequent work showed that LIFU can alter EEG dynamics34. We have also used LIFU to modulate the primary motor cortex (M1)27. We further defined the spatial resolution of LIFU with functional magnetic resonance imaging (fMRI38,39. We have also shown that LIFU is effective for deep brain neuromodulation in humans. We MRI-targeted the ipsilateral thalamus and found LIFU to significantly reduced the amplitude of the P14 SEP generated in VPL of thalamus2.
LIFU mechanisms of action. The mechanism of LIFU is likely due to tissue at the focus point absorbing energy and moving along the direction of the beam. Focused beams cause a distortion of the tissue with subsequent strain that can activate stretch sensitive ion channels causing neurotransmitter release40–43. Kubanek et al. demonstrated mechanosensitive receptors are necessary for ultrasound-elicited behavior in C. elegans44. The expression of Piezo1, a mechanosensitive ion channel, in mammalian cells, but not NaV1.2, imparts ultrasound sensitivity41. The mechanosensitive ion channels TREK-1, TREK-2, TRAAK, and NaV1.5 have all been demonstrated to be activated by ultrasound43,44.
LIFU safety. Ultrasound for neuromodulation follows the safety guidelines of the Food and Drug Administration (FDA) for obstetric diagnostic applications45. The FDA guidelines include derated limits of spatial peak pulse average intensity (ISPPA) of 190 W/cm2, ISPTA of 720 mW/cm2 and a mechanical index (MI = peak negative pressure/ frequency) of 1.9. There are numerous reports on the safety of ultrasound for neuromodulation in small animal9–11,46,47. In a large animal, Dallapiazza looked at hematoxylin and eosin stains of targeted brain regions and did not find any irregularities20. Gaur et al. (2020) found no red blood cell engulfment, no hemosiderin-laden macrophages, no neuronal necrosis and no apoptosis and the examined sections were negative for apoptosis48. In macaques, they found no evidence for any tissue damage at intensities up to 25.8 W/cm2 ISPTA (> 4x what we propose to use here)48. In humans, Stern et al. (2021)49 found no detectable damage using histology at intensities of 5760 mW/cm2 ISPTA that is 8x what we propose. We recently compiled a report of LIFU in humans and found no serious adverse events and a safety profile similar to TMS and TES36.
- Legon, W. et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nature neuroscience 17, 322–329 (2014).
- Legon, W., Ai, L., Bansal, P. & Mueller, J. K. Neuromodulation with single element transcranial focused ultrasound in human thalamus. Human brain mapping 39, 1995–2006 (2018).
- Darmani, G. et al. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clinical Neurophysiology (2021).
- Blackmore, J., Shrivastava, S., Sallet, J., Butler, C. R. & Cleveland, R. O. Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound in Medicine & Biology 45, 1509–1536 (2019).
- Bystritsky, A. & Korb, A. S. A Review of Low-Intensity Transcranial Focused Ultrasound for Clinical Applications. Curr Behav Neurosci Rep 2, 60–66 (2015).
- Sassaroli, E. & Vykhodtseva, N. Acoustic neuromodulation from a basic science prospective. J Ther Ultrasound 4, 17 (2016).
- Naor, O., Krupa, S. & Shoham, S. Ultrasonic neuromodulation. J. Neural Eng. 13, 031003 (2016).
- Kamimura, H. et al. Ipsi- and Contralateral Motor Response Using Ultrasound-induced Neurostimulation in Deeply Anesthetized Mice. Physics Procedia 70, 1212–1215 (2015).
- Kamimura, H. A. S. et al. Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1.9 MHz: FUS neuromodulation of cortical/subcortical brain structures. Med. Phys. 43, 5730–5735 (2016).
- Tufail, Y. et al. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 66, 681–694 (2010).
- Mehi , E. et al. Increased Anatomical Specificity of Neuromodulation via Modulated Focused Ultrasound. PLoS ONE 9, e86939 (2014).
- King, R. L., Brown, J. R., Newsome, W. T. & Pauly, K. B. Effective Parameters for Ultrasound-Induced In Vivo Neurostimulation. Ultrasound in Medicine & Biology 39, 312–331 (2013).
- Kim, H., Chiu, A., Lee, S. D., Fischer, K. & Yoo, S.-S. Focused Ultrasound-mediated Non-invasive Brain Stimulation: Examination of Sonication Parameters. Brain Stimulation 7, 748–756 (2014).
- Yu, K., Niu, X., Krook-Magnuson, E. & He, B. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat Commun 12, 2519 (2021).
- Sharabi, S. et al. Non-thermal focused ultrasound induced reversible reduction of essential tremor in a rat model. Brain Stimulation 12, 1–8 (2019).
- Yang, P. S. et al. Transcranial Focused Ultrasound to the Thalamus Is Associated with Reduced Extracellular GABA Levels in Rats. Neuropsychobiology 65, 153–160 (2012).
- Yoo, S.-S. et al. Focused ultrasound modulates region-specific brain activity. NeuroImage 56, 1267–1275 (2011).
- Yoon, K. et al. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PLoS ONE 14, e0224311 (2019).
- Lee, W. et al. Image-Guided Focused Ultrasound-Mediated Regional Brain Stimulation in Sheep. Ultrasound in Medicine & Biology 42, 459–470 (2016).
- Dallapiazza, R. F. et al. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. Journal of Neurosurgery 128, 875–884 (2018).
- Verhagen, L. et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. eLife 8, e40541 (2019).
- Folloni, D. et al. Manipulation of Subcortical and Deep Cortical Activity in the Primate Brain Using Transcranial Focused Ultrasound Stimulation. Neuron 101, 1109-1116.e5 (2019).
- Kubanek, J. et al. Remote, brain region–specific control of choice behavior with ultrasonic waves. Sci. Adv. 6, eaaz4193 (2020).
- Deffieux, T. et al. Low-Intensity Focused Ultrasound Modulates Monkey Visuomotor Behavior. Current Biology 23, 2430–2433 (2013).
- Wattiez, N. et al. Transcranial ultrasonic stimulation modulates single-neuron discharge in macaques performing an antisaccade task. Brain Stimulation 10, 1024–1031 (2017).
- Yang, P.-F. et al. Neuromodulation of sensory networks in monkey brain by focused ultrasound with MRI guidance and detection. Sci Rep 8, 7993 (2018).
- Legon, W., Bansal, P., Tyshynsky, R., Ai, L. & Mueller, J. K. Transcranial focused ultrasound neuromodulation of the human primary motor cortex. Scientific reports 8, 1–14 (2018).
- Lee, W. et al. Image-Guided Transcranial Focused Ultrasound Stimulates Human Primary Somatosensory Cortex. Sci Rep 5, 8743 (2015).
- Lee, W. et al. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci Rep 6, 34026 (2016).
- Monti, M. M., Schnakers, C., Korb, A. S., Bystritsky, A. & Vespa, P. M. Non-Invasive Ultrasonic Thalamic Stimulation in Disorders of Consciousness after Severe Brain Injury: A First-in-Man Report. Brain Stimulation 9, 940–941 (2016).
- Cain, J. A. et al. Real time and delayed effects of subcortical low intensity focused ultrasound. Sci Rep 11, 6100 (2021).
- Beisteiner, R. et al. Transcranial Pulse Stimulation with Ultrasound in Alzheimer’s Disease—A New Navigated Focal Brain Therapy. Adv. Sci. 7, 1902583 (2020).
- Fomenko, A. et al. Systematic examination of low-intensity ultrasound parameters on human motor cortex excitability and behavior. eLife 9, e54497 (2020).
- Mueller, J., Legon, W., Opitz, A., Sato, T. F. & Tyler, W. J. Transcranial focused ultrasound modulates intrinsic and evoked EEG dynamics. Brain stimulation 7, 900–908 (2014).
- Sanguinetti, J. L. et al. Transcranial Focused Ultrasound to the Right Prefrontal Cortex Improves Mood and Alters Functional Connectivity in Humans. Front. Hum. Neurosci. 14, 52 (2020).
- Legon, W. et al. A retrospective qualitative report of symptoms and safety from transcranial focused ultrasound for neuromodulation in humans. Scientific reports 10, 1–10 (2020).
- Legon, W., Rowlands, A., Opitz, A., Sato, T. F. & Tyler, W. J. Pulsed ultrasound differentially stimulates somatosensory circuits in humans as indicated by EEG and FMRI. PloS one 7, e51177 (2012).
- Ai, L., Mueller, J. K., Grant, A., Eryaman, Y. & Legon, W. Transcranial focused ultrasound for BOLD fMRI signal modulation in humans. in 1758–1761 (IEEE, 2016).
- Ai, L., Bansal, P., Mueller, J. K. & Legon, W. Effects of transcranial focused ultrasound on human primary motor cortex using 7T fMRI: a pilot study. BMC Neurosci 19, 56 (2018).
- Tyler, W. J. The mechanobiology of brain function. Nat Rev Neurosci 13, 867–878 (2012).
- Prieto, M. L., Firouzi, K., Khuri-Yakub, B. T. & Maduke, M. Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz. Ultrasound in Medicine & Biology 44, 1217–1232 (2018).
- Kubanek, J. et al. Ultrasound modulates ion channel currents. Sci Rep 6, 24170 (2016).
- Sorum, B., Rietmeijer, R. A., Gopakumar, K., Adesnik, H. & Brohawn, S. G. Ultrasound activates mechanosensitive TRAAK K + channels through the lipid membrane. Proc Natl Acad Sci USA 118, e2006980118 (2021).
- Kubanek, J., Shukla, P., Das, A., Baccus, S. A. & Goodman, M. B. Ultrasound Elicits Behavioral Responses through Mechanical Effects on Neurons and Ion Channels in a Simple Nervous System. J. Neurosci. 38, 3081–3091 (2018).
- Health, C. for D. and R. Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. U.S. Food and Drug Administration https://www.fda.gov/regulatory-information/search-fda-guidance-documents/marketing-clearance-diagnostic-ultrasound-systems-and-transducers (2019).
- Burks, S. R. et al. Investigation of Cellular and Molecular Responses to Pulsed Focused Ultrasound in a Mouse Model. PLOS ONE 6, e24730 (2011).
- Bystritsky, A. et al. A review of low-intensity focused ultrasound pulsation. Brain Stimulation 4, 125–136 (2011).
- Gaur, P. et al. Histologic safety of transcranial focused ultrasound neuromodulation and magnetic resonance acoustic radiation force imaging in rhesus macaques and sheep. Brain Stimulation 13, 804–814 (2020).
- Stern, J. M. et al. Safety of focused ultrasound neuromodulation in humans with temporal lobe epilepsy. Brain Stimulation 14, 1022–1031 (2021).
- Wager, T. D. et al. An fMRI-Based Neurologic Signature of Physical Pain. N Engl J Med 368, 1388–1397 (2013).
Traumatic Injury
Neurovascular Markers and Inflammatory Predictors in Patients with Traumatic Brain Injury (TBI)
PI: Eric Marvin, DO
Collaboration with Michelle Theus, PhD; and Alicia Pickrell, PhD, Virginia Tech
TBI (traumatic brain injury) affects millions of patients annually, with the most cases being in children, young adults, the elderly, and active-duty military members. Despite how common it is and its ability to cause long-term health problems, our current treatments for TBI are very limited. By gaining a better understanding of the inflammatory (swelling) effects of TBI on the brain, we may identify possible therapeutic targets to reduce inflammation in the brain and cell death after injury. Antiviral drugs and FDA approved compounds that target antiviral proteins in the body called interferons in autoimmune disorders may be able to help TBI patients. Moreover, findings from our study may reveal a new protective marker present in the immune cells of juvenile patients that may be able to be used for therapeutic intervention in adult and aged patients.
In this study, we will study the genetic profile of whole blood and examine whether a virally induced interferon release occurs in humans after TBI. We hypothesize that TBI induces a genetic marker or sign in immune cells induced by circulating interferons in the blood serum of patients after injury depending on the age of the patient.
Additional Information
Traumatic brain injury (TBI) affects 5.3 million Americans and is a leading cause of death and disability; yet, relatively little progress has been made in alleviating neurodegeneration after the initial incident. Inflammation is a key contributor to neuronal death post-injury. When immune cells detect damaged or infected tissue, the Type I interferon response recruits other immune cells. While an inflammatory response is important for clearing damaged tissue debris and infections, sustained inflammation in the brain is harmful. We found that a virally induced interferon response occurs acutely after injury, and genetically knocking out this response in a preclinical model of TBI is protective. Moreover, it is unclear whether this response and the overall transcriptomic response is age dependent. Therefore, our goal is to collect whole blood and evaluate the immune cell genetic profile and interferon presence in pre-adolescent (5-10 years) and adult aged (18-50) male and females human patients following moderate and severe TBI.
We will determine the gene profile of whole blood and interferon/protein expression using serum from patients (5-10 yrs old vs 18-50 yrs old) collected as part of standard care/treatment, usually on at arrival 0-24 hrs (two tubes) and at 48-hrs and 72-hrs (one tube each) post admittance. We will purchase samples for control patients from the University of Virginia repository or commercial sources such as Zenbio. (In order to detect a statistically significant difference in the correlation (if one exists) between age and the absolute value of the interferons at 5% level of significance, 80% power, and small effect size, this study will need 96 patients in eachgroup (children and adults). This will include 96 control adult sera and 96 control child sera to reach statistical significance (total of 192 sera).
The amount or absolute values for inflammatory cytokines; Blood brain barrier junctional proteins; Akap12, and vascular growth or vascular damage factors (all important in the inflammatory cascade process that is triggered in head trauma) will be tested using assays and ELISAs in the laboratory of Dr. Pickrell. Follow-up questionnaires will occur at 1, 3, 6 months and 12 months after discharge as an over the phone follow-up: Glasgow outcome scale (GOS).
Our pre-clinical findings suggest EphA4 is strongly upregulated in the adult brain and blood after TBI. This molecule contributes to tissue damage and prevent the positive effects of Tie2. We also find that younger, pre-adolescent animals are able to suppress EphA4 and prevent progressive damage. When monocytes that lack EphA4 or are from young mice, are transferred to injured adult mice we also find protection after TBI. These studies will confirm our findings in human subjects and help determine if this information can be used for biomarker testing or therapeutics. (EphA4 is a receptor tyrosine kinase that blocks AKT signaling downstream of Tie2 (another tyrosine kinase receptor) to regulate pro-inflammatory polarization state of immune cells. Tie2-expressing monocytes are pro-resolving and are advantageous in injury. Therefore, suppressing or preventing EphA4 activation will improve this pathway, we predict in humans. We do not plan to test integrin and have not mentioned this interaction.
____________
Multi-Modal Biomarkers as Predictors of Post-Traumatic Epilepsy (PTE) and Mild Cognitive Impairment (MCI) after Traumatic Brain Injury (TBI)
PI: Eric Marvin, DO
Collaboration with Michelle Theus, PhD, Virginia Tech
This study aims to determine the relationship between brain blood vessel and brain immune system biomarkers of patients diagnosed with post-traumatic epilepsy (PTE) or mild cognitive impairment (MCI) after traumatic brain injury (TBI) and clinical outcomes. Identifying potential correlations could provide insight into how PTE and MCI develop after TBI and suggest potential pathways for new treatments and clinical management. We will have three participant groups: TBI with PTE, TBI with MCI, and TBI controls. Patients will be recruited for collection of blood samples, as well as some surveys. After collection, samples will be processed for identification of brain blood vessel and immune system biomarkers. Chart review will be conducted for features related to each patient’s demographics, epilepsy, and TBI. All data will be stored in RedCAP and analyzed on SPARC to determine the association between biomarker expression and PTE and biomarker expression and MCI after TBI.
Additional Information
The public health burden of traumatic brain injury (TBI) is substantial, affecting the lives of millions nationwide. Importantly it affects all sex and age groups. A recent report to congress on TBI led to several recommendations for addressing critical gaps. While prevention of TBI is the key public health strategy for reducing the burden, it is imperative for those in public health, clinical practice, and research to design and evaluate effective rehabilitation strategies that reduce the negative health effects of TBI. The report addressed a need for strategies to tackle the short and long-term consequences of head injury. One hallmark of both acute and chronic TBI is neuroinflammation, a known causative agent in the progressive onset of chronic conditions, namely neurodegeneration, post-traumatic epilepsy (PTE), and mental health (PTSD/sleep). Our interdisciplinary approach capitalizes on a focused team aimed at developing common data sets to identify links between neuroimmunology, imaging, PTE, and mild cognitive impairment (MCI) after TBI.
MCI is a common consequence of TBI that is associated with poorer health-related quality of life (HRQoL) scores in physical, social role and emotional functioning, and mental health domains (1). The pathophysiology of MCI after a brain injury is not well understood, but it is associated with a higher risk of further cognitive decline such as Alzheimer’s disease and other dementias (2).
PTE is the development of recurring seizures subsequent to a TBI. PTE accounts for 20% of all epilepsy cases and most commonly affects younger patients (3-5). Most affected patients experience generalized or focal seizures with secondary generalization (6,7). The mechanisms of PTE development are unclear, and there is no known strategy to prevent their onset (8).
To our knowledge, there are no combined clinical-biomarker prognostic models to predict outcomes and progression of PTE or MCI after TBI. Therefore, it is essential to determine the pathophysiology and potential therapeutic targets to prevent their onset or progression.
References
1. Gorgoraptis, N., Zaw-Linn, J., Feeney, C., Tenorio-Jimenez, C., Niemi, M., Malik, A., Ham, T., Goldstone, A. P., & Sharp, D. J. (2019). Cognitive impairment and health-related quality of life following traumatic brain injury. NeuroRehabilitation, 44(3), 321–331. https://doi.org/10.3233/nre-182618
2. LoBue C, Denney D, Hynan LS, Rossetti HC, Lacritz LH, Hart J, Womack KB, Woon FL, Cullum CM. Self-Reported Traumatic Brain Injury and Mild Cognitive Impairment: Increased Risk and Earlier Age of Diagnosis. J Alzheimers Dis. 2016;51(3):727-36. doi: 10.3233/JAD-150895. PMID: 26890760; PMCID: PMC4853649.
3. Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure. 2000;9(7):453-457. doi:10.1053/seiz.2000.0458
4. Jennett B. Trauma as a Cause of Epilepsy in Childhood. Developmental Medicine & Child Neurology. 2008;15(1):56-62. doi:10.1111/j.1469-8749.1973.tb04866.x
5. Semah F, Picot MC, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51(5):1256-1262. doi:10.1212/WNL.51.5.1256
6. Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Archives of Physical Medicine and Rehabilitation. 1997;78(8):835-840. doi:10.1016/S0003-9993(97)90196-9
7. Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: A prospective, multicenter investigation. Archives of Physical Medicine and Rehabilitation. 2003;84(3):365-373. doi:10.1053/apmr.2003.50022
8. Agrawal A, Timothy J, Pandit L, Manju M. Post-traumatic epilepsy: An overview. Clinical Neurology and Neurosurgery. 2006;108(5):433-439. doi:10.1016/j.clineuro.2005.09.001
____________
Predictive Markers for Recovery in CSF of Spinal Cord Injury Patients
PI: Adeolu Olasunkanmi, MD
Collaboration with Pam VandeVord, PhD, Virginia Tech
Spinal cord injury is a complicated and devastating neurological disease. Initial primary injury is often worsened by the bodies’ natural recovery systems due to the reckless nature of some healing responses causing a dangerous cycle of secondary, and further, injuries, such as large bleeds, blood clots, constriction of important blood vessels, stroke, and swelling on the spinal cord (1). The occurrence of these secondary injuries worsen the initial trauma injury with additional and growing damage to nearby spinal cord tissue leading to further cell death of neurons and their protective cells, the movement of harmful inflammatory cells to the area, as well as the condition, astrocytosis which limits secondary injury but also slows the regeneration of new healthy neurons (1). Indeed, spinal cord injury is a major cause of long-term physical impairment or disability in the United States with a new-case rate of approximately 54 cases per one million persons or 17,700 cases annually (2). Moreover, the present number of cases of spinal cord injury in the United States is 288,000 persons (2). Current treatments are limited to early surgical intervention, strict blood pressure control, and basic supportive measures. In recent years, numerous studies have begun to investigate the important biological processes negatively impacted by spinal cord injury in order to develop novel therapeutics to target the specific molecules involved in those processes that may be able to prevent secondary injury. However, the majority of these potential molecular targets have only been identified in animal models (2). To our knowledge, no study to date has investigated human cerebrospinal fluid or genetic biological markers at different time points in patients with various types of trauma-related spinal cord injuries. The primary aim of the present study is to evaluate cerebrospinal fluid inflammatory profiles and genetic markers in patients with various types of acute spinal cord injury in order to predict degree of potential harm to the nervous system as well as identify theoretical molecular targets for new therapeutic medications.
In conjunction with the collection of cerebrospinal fluid for analysis, a secondary aim of the present study is to evaluate the effectiveness of draining cerebrospinal fluid for a total of 72 hours on the healing of the neurologic injury. Prior studies have supported the use of cerebrospinal fluid draining as an additional preventative intervention of lower body paralysis in patients undergoing a type of spinal cord injury-related aneurysm’s corrective surgery (6). Indeed, animal studies evaluating the use of cerebrospinal fluid draining have reported 12% of patients developing lower limb neurologic deficits who underwent cerebrospinal fluid draining compared to 33% of control subjects (without cerebrospinal fluid draining) during this surgery.
In the present study, we will gain these data and implement cerebrospinal fluid drainage in order to keep intrathecal pressure at a goal of 10mm Hg at all times. As such, placement of a lumbar (lower spine) drain will serve a dual purpose. First, it will serve as a way where cerebrospinal fluid can be collected without repeated lumbar punctures over the course of 72 hours. Second, it will allow for cerebrospinal fluid drainage to keep pressures low to assess for neurologic improvement.
____________
Retrospective Study of Traumatic Spinal Cord Injury
PI: Adeolu Olasunkanmi, MD
Spinal cord injury is a complicated and devastating neurological disease. Initial primary injury is expanded by ongoing pathologic disruption causing secondary damage, such as hemorrhage, intravascular thrombosis, vasospasm, ischemia, and spinal cord edema (1). Cascades of secondary injury expand the initial injury with progressive damage to adjacent tissue fostering further neuronal and glial death, migration and proliferation of reactive inflammatory cells, as well as reactive astrocytosis, which diminishes the expanse of secondary injury but simultaneously inhibits axonal regeneration (1). Indeed, spinal cord injury represents a major cause of long-term physical impairment in the United States with an incidence of approximately 54 cases per one million persons or 17,700 cases annually (2). Moreover, the prevalence of spinal cord injury in the United States is 288,000 persons (2). Current treatments are limited consisting of early surgical decompression and stabilization, strict blood pressure control, and supportive measures. In recent years, numerous studies have begun to investigate the molecular pathophysiology of spinal cord injury in order to develop novel therapeutics to target molecular mediators of secondary injury. However, the majority of these potential molecular targets have been identified in animal models (2). To our knowledge, no study to date has investigated cerebrospinal fluid inflammatory profiles or genetic markers at several time points in patients with various types of acute spinal cord injury with a multivariate analysis. The aim of the retrospective study is to evaluate instance of traumatic spinal cord injury and neurological outcomes at follow up as a control for our proposed prospective study to investigate cerebrospinal fluid inflammatory profiles and genetic markers in patients with various types of acute spinal cord injury.
A retrospective study will be implemented where charts will be reviewed from January 1 2014 – September 1, 2024 for all traumatic spinal cord injury patients The subject population will consist of all male and females indiscriminate of race or ethnicity, who are admitted to Carilion Roanoke Memorial Hospital for acute spinal cord injury of cervical or thoracic spine.
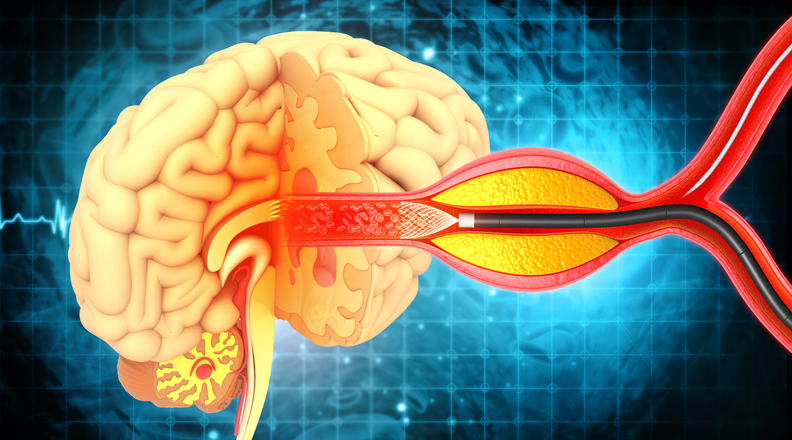
Vascular
Interrogating Human Serum Expression of EphA4 and Collateral Vessel Function Following Acute Ischemic Stroke
PI: Biraj Patel, MD
Collaboration with Michelle Theus, PhD, Virginia Tech
Stroke is the fifth leading cause of death and represents one of the leading causes of permanent neurological disability in the United States, afflicting nearly 800,000 people annually. To date, there are no safe and effective therapies to promote brain tissue stability and recovery. The current proposal seeks to extend novel pre-clinical animal findings into human clinical studies by evaluating specialized vessels in the brain called collaterals. Collateral vessels are natural “bypass” vessels that exist in our brain which lie dormant but following blockage of a major artery they act as alternative routes for blood to travel through which helps maintain flow of oxygen and nutrients. Patients with good collateral function recover better than those with poor function, however, the reason for this difference remains unknown. Our rodent studies indicate EphA4 receptor can prevent collateral growth and its release into the circulation may be a biomarker or sign of collateral function after stroke. This proposal will compare the serum levels of EphA4 with patient collateral scores on CT and other conventional techniques to view blood flow, as well as tissue damage using CT perfusion to view the circulation of blood in the brain (when applicable). These findings will improve our understanding of patient collateral plasticity and provide evidence for targeting EphA4 for predictive and therapeutic interventions.
Although improvements in wide-spread community awareness regarding the symptoms of stroke (ex/BE-FAST; balance, eyes, face, arm, speech and time) have prompted more rapid hospital admittance, close to half of all patients either do not arrive within 3 hours for tPA therapy which must be used early for success or they present with large vessel blockages for which tPA is ineffective. Recently, with the advent of endovascular therapy (ex/ clot removal using endovascular mechanical embolectomy or surgically removing endovascular blood clots), tissue protection can be achieved beyond the traditional 3-hour best treatment window and up to 24 hours post-symptom onset [4, 5]. The cerebral collateral circulation represents a powerful determinant of ischemic stroke outcome and enhancing this response through collateral therapeutics is under intense investigation. Moreover, it remains unclear why greater collateral function exists in some patients compared to others.
Additional Information
Our extensive pre-clinical findings in rodents show that vessel surface level cell-specific genetic Deletion of EphA4 receptor tyrosine kinase improves collateral function, enhances cerebral blood flow and provides protection against stroke within 24-hours. We also show that continuous, systemic delivery of EphA4 peptide inhibitor KYL EphA4 specifically can be found in EV released from cultured cells. The current proposal seeks 1-year support to test serum and serum-derived exosome for the presence of EphA4 in patients following stroke compared to control patients using human EphA4 ELISA kit (MyBioSource, Inc). These levels of EphA4 will be correlated with each patient collateral score using CT and conventional angiography using appropriate statistical analysis. We predict to find the highest levels of EphA4 in the serum of patients with least collateral function which will correlate with greater tissue damage. Levels of Angiopoeitin-1 and Angiopoeitin-2 will also be measured via ELISA to determine protein expression. This project focuses on identification of protein expression via ELISA without genetic analysis.
____________
Stroke Thromboembolism Registry of Imaging and Pathology (STRIP)
PI: Biraj Patel, MD
With the advent of mechanical thrombectomy, a novel surgical technique in which blood clots are removed by going directly through the blood vessels with thin tubes, for the treatment of acute ischemic stroke, there has been growing interest in the cellular and imaging characteristics of retrieved thromboemboli, resected blood clot tissues. Prior studies have suggested that studying clot composition can 1) provide insights into stroke causal factors and risk factors and 2) demonstrate blood flow restoration success with intravenous thrombolysis and mechanical thrombectomy. Furthermore, with improved characterization of clot composition regarding imaging and histopathologic features, it is possible that the imaging characteristics of a clot could help in device selection prior to the mechanical thrombectomy procedure itself. The goal of this study is to evaluate the clots that are extracted from a patient's brain during a stroke. Specifically, looking at what the clot is made up of and how it related to the ease of extraction, imaging, and patient outcome.
____________
Elucidating potential role and dynamics of cellular transmembrane protein expression in aneurysmal subarachnoid hemorrhage patients and associations with symptomatic vasospasm
PI: Kristine Ravina, MD
Collaboration with Scott Johnstone, PhD, Virginia Tech
Aneurysmal subarachnoid hemorrhage (aSAH) induced vasospasm carries high risk of delayed cerebral ischemia in about 30% of patients that can potentially lead to neurologic deficits, permanent disability, and mortality representing a major healthcare burden. aSAH vasospasm has been associated with inflammatory processes in the subarachnoid space, however, the underlying cellular mechanisms are poorly understood. Blood products and vessel wall injury have been shown to trigger macrophage recruitment around the time of aSAH vasospasm. During our preliminary feasibility analysis, we collected tips of devices used in cerebral angiograms and endovascular interventions and processed them for RNA in the Johnstone Lab at Fralin Biomedical Research Institute at Virginia Tech Carilion. We were able to detect signal for housekeeping gene B2m as well as macrophage marker CCR2 and endothelial cell marker CDH5 via qPCR. This data indicates that cellular material including macrophages and endothelial cells is present on the endovascular devices and thus can be used to further study the role of immune cells in aSAH. Considering recent evidence that macrophages can communicate with other cell types and modulate their functions via gap junctions (GJ) - cell-to-cell communications made of connexin proteins such as Cx43 -, we hypothesize that recruited macrophages may be directly communicating with vascular smooth muscle cells after aSAH to modulate their contractile responses leading to the development of vasospasm. To test this hypothesis, we will study immune cell burden, Cx43 and contractility marker expression dynamics in aSAH patient cerebrospinal fluid (CSF) and endovascular device tissue and their associations with vasospasm indices and clinical outcomes. This study has the potential to provide the first direct human evidence of the Cx43-GJ communication control of aSAH vasospasm opening avenues for novel vasospasm marker and therapeutic development.
The potential clinical benefits of the proposed study are two-fold: 1) developing a basis for early detection of vasospasm and follow-up of aSAH treatment responses via determination of immune cell-Cx43-GJ signaling status in CSF and endovascular device material, and 2) Identifying a novel therapeutic strategy for aSAH vasospasm treatment via modulation of Cx43-GJ cell-cell communication.
Carilion Clinic treats ~60 aSAH patients yearly. Nearly all of them undergo endovascular intervention for aneurysm diagnostics and/or treatment and majority require external ventricular drain (EVD) placement. We will enroll and consent patients with confirmed aSAH who require external ventricular drain (EVD) placement for CSF diversion. The group assignment will occur either when a subject has a vasospasm event recorded during the first 10 days of their hospitalization (enrolled in vasospasm group) OR on day 10 of their hospitalization if they have not had a vasospasm event recorded (non-vasospasm group). Study members will be reviewing enrolled subject charts daily to determine whether or not there is a vasospasm event recorded and will perform study activities accordingly. All aSAH patients will be treated following the standard of care, including close monitoring in the intensive care unit for up to 21 days, daily nimodipine treatment, EVD placement in cases of hydrocephalus, and daily transcranial Doppler flow velocity (TCD) assessments. The power analysis with an effect size of 0.2 and significance level of 0.05 determined that a total sample size of at least 36 patients (18 per group) is needed to achieve the desired power of 0.8. All adult patients with aSAH treated at Carilion Clinic will be considered for inclusion in the proposed study regardless of their sex or age. No vulnerable groups will be excluded from the study and participants will be considered for inclusion solely based on proposed criteria.
____________
The Effect of Dextrose 5% in 0.225% Sodium Chloride (D5 ¼NS) on Postoperative Chronic Subdural Hematoma Size and Recurrence Rate: a Prospective, Randomized, Double-blinded, Placebo Controlled Clinical Trial
PIs: Kristine Ravina, MD, and Eric Marvin, DO
How can we improve recurrence rates of chronic subdural hematomas to reduce need for surgical intervention? We hypothesize that Dextrose 5% in 0.225% Sodium Chloride (D5 ¼NS) will increase the intracellular brain volume and water content post-operatively, reducing the residual subdural space that is created by evacuation of the chronic subdural hematoma (CSDH), and that this, in turn, will reduce the hematoma volume that will then potentially lead to improved functional outcomes, reduced hospitalization time as well as the reduced rate of symptomatic recurrence requiring redo surgery.
Additional Information
Chronic subdural hematoma (CSDH) is a neurological disease characterized by a collection of fluid, blood, and blood degradation matter between the arachnoid and dura mater in a well developed membrane cavity (Edlmann, Giorgi-Coll, Whitfield, Carpenter, & Hutchinson, 2017). The average age of onset of CSDH is 63 years and the effects of aging on the brain play a role in 1) susceptibility of CSDH and 2) the ability of the brain to re-expand, thus affecting the overall rate of volume change in residual CSDH (De Bonis et al., 2013; Peters, 2006). With some exceptions, burr-hole evacuation and craniotomies are typically the first line surgeries used to evacuate CSDHs (Matsumoto et al., 2018). However, many neurosurgeons practice based on their own experiences and beliefs in effort to decrease the recurrence rate of CSDH and patient morbidity, resulting in multiple different surgical interventions. Evidently, one of the significant problems that CSDH poses is the high rate of recurrence, which currently stands between 7 and 30% worldwide (Zhang, Chen, Xiao, & Tang, 2017). The recurrence of a CSDH is typically defined as the presence of residual or recurrent CSDH after the first surgery, leading to additional surgical intervention either within 3 months (early recurrence) or after 3 months (late recurrence) (Oh et al., 2010). Factors leading to recurrent CSDH include age, use of anticoagulant therapy, volume of hematoma cavity, degree of midline shift on CT, presence of residual air post-operatively, and volume of residual hematoma fluid (Motiei-Langroudi et al., 2017). Post-operatively, the current body of literature has supported the use of a drain, patient positioning (supine), and aggressive administration of crystalloid IV fluids as ways to facilitate brain re-expansion and decrease formation of recurrent CSDH (Yadav, Parihar, Namdev, & Bajaj, 2016). The subdural space, in most people, is a “potential” space, with close opposition of the arachnoid of the cerebral convexities and the inner surface of the dura. A CSDH creates a real space between the arachnoid and the inner layer of the dura. If this space is not filled with the re-expanded brain, fluid will inevitably fill it. As the brain re-expands, any residual fluid secondary to the surgical intervention or residual/recurrent CSDH is able to escape via the drain, which has shown to reduce recurrence (Komotar, Starke, & Connolly, 2010). Patient positioning has proved to be an effective measure for allowing brain re-expansion. The concept is somewhat contradictory to most neurosurgical pathologies, such as an acute subdural hematoma, in which patients are placed in a 30-45 head of bed elevation position (Feldman et al., 1992). The supine position encourages increased blood flow to the brain, decreased drainage of venous blood to the heart and cerebrospinal fluid (CSF) drainage to the lumbar thecal cisterns, by removing gravity from the physiologic process. This is thought to increase intracranial pressure (ICP), expand the volume of the brain, and decrease the volume of the subdural space (Abouzari et al., 2007). The use of IV fluids acts on the same principle by re-expanding vascular volume and increasing cerebral blood flow to facilitate brain re-expansion (Janowski & Kunert, 2012; Yadav et al., 2016). Studies have focused on the use of solely crystalloid fluids, mainly normal saline (NS) (Smorenberg, Ince, & Groeneveld, 2013). Although the literature has shown the beneficial effects of these interventions, a high level of variability exists in surgeons’ operative and post-operative care, which tries to address the multiple factors that lead to recurrence. This illustrates the difficulty in trying to reduce post-operative recurrence.
Typically, osmolarity is not included in the discussion of IV fluids and their role in brain re-expansion for CSDH treatment; the literature focuses on the administration of IV fluids, but not the quantitative characteristics of these fluids. Normal human reference range of serum osmolarity is 275 – 299 mOsm/kg (Hooper et al., 2015). The osmolarity of NS is 308 mOsm/kg, which is considered to be isotonic to plasma. While NS is the only IV fluid used post-operatively in CSDH, other IV fluids that are more hypotonic to plasma may be more potent at reducing the residual CSDH space and therefore reducing recurrence. As stated before, there is a relationship between the residual hematoma fluid (which can be indicative of the volume of that space) and recurrence rate (Motiei-Langroudi et al., 2017), so reducing this space and potential area for fluid to collect may be beneficial in reducing the rate of recurrence. D5 1/4NS is comprised of 5% dextrose in a 0.225% sodium chloride (NaCl) solution. D5 1/4NS is initially slightly hypertonic to plasma, with an osmolarity of 329 mOSm/L (Trissel, 2012). As the glucose component is rapidly metabolized, 0.225% NaCl solution remains and is hypotonic to plasma at an osmolarity of 77 mOsm/L (Alvis-Miranda, Castellar-Leones, & Moscote-Salazar, 2014). Because of the hypotonicity of the 1/4NS, it shifts into the intracellular compartments, followed by free water, and allows for volume expansion. Therefore, the administration of D5 1/4NS secondary to its inherent fluid dynamic properties, will facilitate brain re-expansion (Montano, Stifano, Skrap, & Mazzucchi, 2017). Hypotonic solutions are used in neurosurgical patients to treat hypernatremia, to address free water deficits, and to flush after tube feeds for example, therefore we believe that this IV therapy is safe in neurosurgical patients (Dickerson et al., 2013). As an added benefit, the 1/4NS provides both sodium and chloride to help address electrolyte imbalances that may have been exacerbated by the administration D5W, for example. We believe that the use of D5 1/4NS can address the gap in knowledge that persists in the treatment of CSDH: is there a therapy that can reduce recurrence as defined by the need for further surgical intervention therefore preventing the inherent increased risks for post-surgical complications associated with drain placement and patient positioning? Our study will help determine whether D5 1/4NS is a significantly better brain volume expander than NS, measured by residual/recurrent hematoma volume at 24h post-surgery and assessing other measures such as the length of hospitalization, functional outcomes and recurrence rate needing redo surgical intervention which is defined as a second surgical intervention within a 90-day period (+/- 7 days) after the initial surgery.
____________
Pericytes in Germinal Matrix Hemorrhage
PI: Lisa Apfel, MD
Collaboration with John Chappell, PhD, Virginia Tech
Intraventricular hemorrhage (IVH), or germinal matrix hemorrhage (GMH), which are brain bleeds included in a range of conditions that contribute to brain damage in babies born prematurely. These pathologies can have a devastating impact on brain development over an individual’s lifetime. It is critical to expand the approaches and biomarkers that can (i) inform infant risk for developing these conditions and associated secondary injuries, (ii) assess infant recovery and effectiveness of clinical care, (iii) monitor patient condition, and (iv) inspire the development of a therapeutic target that could prevent or decrease brain damage for premature infants, particularly those of very low birth weight.
Additional Information
Previous studies have explored the clinical utility of assessing concentrations of certain molecular factors in the cerebrospinal fluid (CSF) of IVH-GMH patients to stratify them according to injury severity and the need for additional interventions such as shunting after post-hemorrhagic ventricular dilation (PHVD) (1). However, to our knowledge, very few studies, if any, have thoroughly analyzed clinical samples to adequately address the potential utility of cerebrovascular-based biomarkers of intraventricular hemorrhage (IVH) and post-hemorrhagic ventricular dilation (PHVD), specifically markers associated with capillary pericyte investment and integrity of the extracellular matrix (ECM) that comprises the cerebral vascular basement membrane.
Pericytes are highly abundant within the brain microcirculation and are associated with the maintenance of cerebrovascular integrity and blood-brain barrier (BBB) function (3). Pericyte integration and retention within the brain vasculature depends, in part, on regulation of signals within the Platelet-Derived Growth Factor-BB (PDGF-BB) pathway (4). We and others have recently found a soluble isoform of the primary PDGF-BB signaling receptor on pericytes, PDGF Receptor-beta (PDGFRb) (5-6). Soluble PDGFRb (sPDGFRb) likely tethers to the cerebrovascular ECM to regulate PDGF-BB signaling, and its abundance in the CSF may be indicative biomarker of neurovascular trauma and/or dysfunction that is a consequence of, or perhaps drives, IVH-GMH pathogenesis. This hypothesis has been tested in the context of Alzheimer’s Disease (7), but little to no evidence has been collected for its relevance to the setting of the perinatal brain and encephalopathy of prematurity conditions in particular.
Furthermore, because sPDGFRb binds ECM components via heparan sulfate binding domains, degradation of the neurovascular basement membrane during IVH-GMH onset and progression may yield additional biomarkers of disease severity and resolution. In particular, Type IV collagen (col-IV), laminins (e.g. laminin-211), and vitronectin have been associated with pericyte investment (8), and these ECM elements are likely fragmented if pericytes detach and/or microvessels are damaged or ruptured. ECM fragments have been tested in other neurological disorders such as idiopathic normal pressure hydrocephalus (9), but few studies have explored the detection of ECM fragments in the CSF of IVH-GMH patients to stratify disease severity and identify any correlations with morphological and neurological outcomes.
The aim of the present study is to determine whether altered levels of molecular cues involved in pericyte investment (e.g. regulators within the platelet-derived growth factor-BB pathway, specifically sPDGFRb) or extracellular matrix integrity (e.g. Type IV Collagen, laminin-211, vitronectin) correlate with clinical variables early in the disease course of IVH with or without periventricular hemorrhagic infarction (PHI) and PHVD. Identifying potential correlations could provide insight into the pathogenesis of these conditions and suggest candidate therapeutic targets for their clinical management. Moreover, potential biomarkers may emerge that could provide a more comprehensive assessment of each patient and stratify their risk for developing these conditions and any associated complications. This could in turn guide clinical care, specifically selecting appropriate treatment options as well as the time-course for their implementation.
- Douglas-Escobar M, Weiss MD. Biomarkers of brain injury in the premature infant. Front Neurol. 2013 Jan 22;3:185. doi: 10.3389/fneur.2012.00185. PMID: 23346073; PMCID: PMC3551194.
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009 Jan;8(1):110-24. doi: 10.1016/S1474-4422(08)70294-1. PMID: 19081519; PMCID: PMC2707149.
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010 Nov 25;468(7323):562-6. doi: 10.1038/nature09513. Epub 2010 Oct 13. PMID: 20944625; PMCID: PMC3241506.
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005 Sep 16;97(6):512-23. doi: 10.1161/01.RES.0000182903.16652.d7. PMID: 16166562.
- Darden J, Payne LB, Zhao H, Chappell JC. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis. 2019 Feb;22(1):167-183. doi: 10.1007/s10456-018-9648-z. Epub 2018 Sep 20. PMID: 30238211; PMCID: PMC6360133.
- Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett. 2015 Oct 21;607:97-101. doi: 10.1016/j.neulet.2015.09.025. Epub 2015 Sep 25. PMID: 26407747; PMCID: PMC4631673.
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019 Feb;25(2):270-276. doi: 10.1038/s41591-018-0297-y. Epub 2019 Jan 14. PMID: 30643288; PMCID: PMC6367058.
- Zhao H, Chappell JC. Microvascular bioengineering: a focus on pericytes. J Biol Eng. 2019 Mar 29;13:26. doi: 10.1186/s13036-019-0158-3. PMID: 30984287; PMCID: PMC6444752.
- Minta, K., Jeppsson, A., Brinkmalm, G. et al. Lumbar and ventricular CSF concentrations of extracellular matrix proteins before and after shunt surgery in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 18, 23 (2021). https://doi.org/10.1186/s12987-021-00256-1
____________
Pilot Study Establishing the Maternal, Fetal, and Placental Contributions to Circulating Soluble Platelet-Derived Growth Factor Receptor-beta (sR-beta) during Gestation
PI: Jordan Darden, PhD
Collaboration with John Chappell, PhD, Virginia Tech
Soluble Platelet-Derived Growth Factor Receptor-beta (sPDGFR-beta, or sR-beta) isoforms have recently been identified as variants of the full-length PDGFR-beta receptor found on pericytes and vascular smooth muscle cells (Payne et al. Biomolecules 2023). Recent clinical data reported these isoforms to be present in the cerebrospinal fluid (CSF) of adult humans, specifically in the context of dementia and neurological impairment (Nation et al. Nat Med 2019). Blood contents contribute to CSF composition, but levels of sR-beta were not measured in patient blood in this references study. We have found sR-beta to be abundant in mouse blood (Payne et al. Biomolecules 2023), motivating in part our pilot study currently at Carilion Clinic. The pilot study has established the presence of these sR-beta isoforms in the umbilical cord blood of newborns, but the sources of these variants remain to be identified. The study proposed here is aimed at determining: (1) the presence and amount of sR-beta in maternal blood in the overall process of gestation (the 9 months of being pregnant) and at the time of delivery, (2) corresponding levels of sR-beta in umbilical cord blood, and (3) the presence and amount of sR-beta protein and mRNA splice variants in the associated placenta. To establish these baseline clinical values, we seek to enroll pregnant mothers without anomalies who will be delivering via a scheduled repeat cesarean section at full-term. Maternal blood will be collected from a standard of care blood draw at the time of delivery to measure sR-beta levels in the serum. Umbilical cord blood will be collected alongside blood typing samples, to analyze fetal blood levels of sR-beta. Placental tissue samples will be collected and analyzed for sR-beta protein levels, sR-beta mRNA levels and localization, and general histopathology.
Additional Information
Platelet-Derived Growth Factor-BB (PDGF-BB) is an essential signaling molecule for numerous processes underlying fetal growth and development, with critical roles in blood vessel formation, among other organ systems (Andrae et al. Genes Dev 2008). PDGF-BB can stimulate the growth, migration, and survival of several cell types including vascular smooth muscle cells (vSMCs) and capillary pericytes (PCs), which all express full-length PDGF Receptor-beta (PDGFR-beta) (Hellstrom et al. Development 1999). Recently, truncated isoforms of PDGFR-beta have been identified that may arise from enzymatic cleavage (Sagare et al. Neurosci Letters 2015) or from mRNA alternative splicing (Payne et al. Biomolecules 2023). These variants appear to be soluble (sPDGFR-beta, or sR-beta) with relatively high abundance in various murine tissues including the brain, kidney, and blood (Payne et al. Biomolecules 2023). They have also been detected at abnormally high levels in the cerebrospinal fluid (CSF) of human patients with dementia and/or Alzheimer’s Disease (Nation et al. Nature Medicine 2019). Recent unpublished data from the Chappell lab has detected sR-beta isoforms in human serum of blood drawn at the time of delivery from umbilical cords alongside standard of care collections for blood typing. The source of these sR-beta variant is currently unknown, and establishing baseline values for their levels in fetal, maternal, and placental tissues will allow for comparison with scenarios where pregnancies may involve specific complications such as preeclampsia.
The goal of this study is to establish the presence and levels of sR-beta in maternal, fetal and placental tissue compartments as well as to determine the cellular source of sR-beta protein and its alternatively spliced mRNA transcripts. Maternal and umbilical cord blood will be collected, and the associated serum fraction will be analyzed for sR-beta levels. Transcriptional and protein analysis of placental tissue samples will be conducted in conjunction with histopathological localization of sR-beta isoform transcripts relative to placental cell types including vascular and immune cells.
____________
Investigating the Effects of Placental Cerebrovascular Malperfusion on the Development of Hydrocephalus, Intraventricular Hemorrhage, and Periventricular Leukomalacia in Pre-Term Infants in Rural Appalachia
PI: Jordan Darden, PhD
Access to specialized neurosurgical care is challenging globally, particularly in rural and underserved regions like Appalachia. Conditions such as hydrocephalus, periventricular leukomalacia (PVL), and germinal matrix hemorrhage, also called intraventricular hemorrhage (IVH), in premature infants often go underdiagnosed and undertreated due to these disparities. Current treatments for these cerebrovascular complications are limited and largely reactive, demonstrating the need for improved, proactive approaches. This study proposes a retrospective chart review to investigate the relationship between placental pathology and cerebrovascular outcomes in preterm infants, focusing on maternal vascular malperfusion and other placental factors potentially linked to PVL, IVH, and hydrocephalus. Our primary objective is to identify key risk factors and patterns associated with these conditions, hypothesizing that placental health plays a significant role in neonatal cerebrovascular outcomes. Existing noninvasive techniques lack the specificity and depth required to fully understand these underlying mechanisms, so this approach aims to overcome these limitations by gathering comprehensive data from neuroimaging, placental histopathology, and patient demographics. In the long term, this research could lay the foundation for grant-funded prospective studies, enabling the development of targeted interventions to improve neonatal outcomes and reduce the burden of neurological complications in vulnerable populations with limited healthcare access.
Additional Information
Infant prematurity is defined as the birth of a child before they have reached full term at 38 weeks of gestation. There are varying levels of prematurity, which are closely related to the week the infant was born. Typically, most viable premature births without long-term deficits occur after 28 gestational weeks, with rare cases of infants surviving before 24 weeks. (Koh et al., 2000). There are multiple causes of premature birth, including diabetes, infection, and high blood pressure. However, there has been an increasing amount of literature that suggests malperfusion of the placenta can lead to premature births—furthermore producing an underdeveloped germinal matrix, a region near the ventricles that is extensively vascularized and fragile in preterm infants. This increases the chances of intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and the further development of hydrocephalus due to bleeding within the structures of the brain.
IVH is categorized as bleeding within the ventricles of the brain due to the underdevelopment of the vasculature, leading to fragile vessels susceptible to rupture. PVL is the degeneration of white matter tracts that line the ventricles within the brain, mainly due to a lack of perfusion to these structures characterized by prematurity. It is often closely associated with IVH. Furthermore, it has been brought to focus that a potential cause of hydrocephalus in preterm infants may be due to the breakdown of the vascular placental connection between mother and infant (Robinson, 2012).
According to Stanford Medicine, approximately 1 in every 500 babies in the United States is born with hydrocephalus, making it more common than Down's syndrome, spina bifida, or brain tumors (About Pediatric Hydrocephalus, 2024). Unfortunately, there are no specific statistics currently available for the rate of hydrocephalus in the Appalachian region; the incidence can be presumed to be significant as much of the area is rural with limited healthcare access. Hydrocephalus is a substantial cause of pediatric brain surgery and requires lifelong management, often involving surgical interventions like shunt placements or ventriculostomies to control cerebrospinal fluid buildup. Due to its geographical location, populations served by Carilion Clinic frequently encounter barriers to care, delaying diagnoses and further treatment of neurological ailments.
These gaps in healthcare are notably concerning for hydrocephalus, as timely intervention can be the difference between short-term and lifelong disparities. Hydrocephalus, characterized by abnormal brain swelling due to cerebrospinal fluid accumulation, can arise from various insults, either as a primary condition or secondary to complications such as periventricular leukomalacia (PVL) or intraventricular hemorrhage (IVH) in premature infants. Recent studies suggest there may be a relationship between the cerebrovascular connection between the placenta and the mother. Furthermore, we suspect these conditions are especially prevalent in geographically vulnerable populations. To improve patient outcomes, it is imperative to investigate the underlying biological causes and progression of these conditions, helping clinicians understand the distinct challenges in accessing care for hydrocephalus and its associated conditions.
A brief overview of the literature demonstrates that maternal vascular malperfusion is heavily associated with intraventricular hemorrhage (IVH) in preterm infants. Furthermore, they indicate the importance of studying placental pathology in neonatal outcomes. There have been conflicting results from multiple studies that initially linked chorioamnionitis to IVH, with some saying there is a weak association upon further analysis(Oh et al., 2021) and others saying there is a notable correlation (Villamor-Martinez et al., 2018), (Harteman et al., 2012),(Koschnitzky et al., 2018),(Çaksen et al., 2021). Angiogenic inhibitors show promise in stabilizing vasculature to prevent IVH (Deger et al., 2021), whereas gene-environment interactions may increase hemorrhage risk (Ment et al., 2015). Additionally, inflammation and coagulation disorders contribute to vascular damage and IVH, with cytokines like IL-1β and TNF-α implicated in disrupting cerebral blood flow (Ment et al., 2015). Placental underreporting and mixed findings on lesions like thrombosis, infarction, and intrauterine infections suggest that IVH's risk factors are more complex (Catov et al., 2017). These insights highlight the need for further research on the role of placental health in IVH and related neonatal complications. Additionally, infants with IVH are at a significant risk of developing PVL. This condition is believed to be primarily caused by changes in blood flow to the fragile area around the brain's ventricles, especially before 32 weeks of gestation (Starr et al., 2024).
Our research aims to explore the prevalence, characteristics, and treatment patterns of hydrocephalus, PVL, and IVH within our current patient population at Carilion Clinic. By focusing on this data set, we hope to gain valuable insights into the specific needs and challenges these patients face, creating the potential to determine targeted strategies to help improve the relationship between healthcare access and outcomes for hydrocephalus, PVL, and IVH. Ultimately, we aim to build a holistic foundation geared towards preventing the need for invasive neurosurgical care within this community and, hopefully, in communities across the nation.
- About Pediatric Hydrocephalus. (2024). Stanford Medicine Children’s Health. https://www.stanfordchildrens.org/en/services/hydrocephalus/about.html
- Çaksen, H., Köseo lu, F. T., Güven, A. S., Altunhan, H., yisoy, M. S., & Aç kgözo lu, S. (2021). Risk and Prognostic Factors in Perinatal Hemorrhagic Stroke. Annals of Indian Academy of Neurology, 24(2), 227. https://doi.org/10.4103/aian.AIAN_580_20
- Catov, J. M., Scifres, C. M., Caritis, S. N., Bertolet, M., Larkin, J., & Parks, W. T. (2017). Neonatal outcomes following preterm birth classified according to placental features. American Journal of Obstetrics and Gynecology, 216(4), 411.e1-411.e14. https://doi.org/10.1016/j.ajog.2016.12.022
- Deger, J., Goethe, E. A., LoPresti, M. A., & Lam, S. (2021). Intraventricular Hemorrhage in Premature Infants: A Historical Review. World Neurosurgery, 153, 21–25. https://doi.org/10.1016/j.wneu.2021.06.043
- Harteman, J. C., Nikkels, P. G. J., Kwee, A., Groenendaal, F., & de Vries, L. S. (2012). Patterns of placental pathology in preterm infants with a periventricular haemorrhagic infarction: Association with time of onset and clinical presentation. Placenta, 33(10), 839–844. https://doi.org/10.1016/j.placenta.2012.06.014
- Koh, T. H. H. G., Harrison, H., & Casey, A. (2000). Prediction of survival for preterm births: Survival table was not easy to understand. BMJ : British Medical Journal, 320(7235), 647.
- Koschnitzky, J. E., Keep, R. F., Limbrick, D. D., McAllister, J. P., Morris, J. A., Strahle, J., & Yung, Y. C. (2018). Opportunities in posthemorrhagic hydrocephalus research: Outcomes of the Hydrocephalus Association Posthemorrhagic Hydrocephalus Workshop. Fluids and Barriers of the CNS, 15(1), 11. https://doi.org/10.1186/s12987-018-0096-3
- Ment, L. R., Ådén, U., Bauer, C. R., Bada, H. S., Carlo, W. A., Kaiser, J. R., Lin, A., Cotten, C. M., Murray, J., Page, G., Hallman, M., Lifton, R. P., & Zhang, H. (2015). Genes and Environment in Neonatal Intraventricular Hemorrhage. Seminars in Perinatology, 39(8), 592. https://doi.org/10.1053/j.semperi.2015.09.006
- Oh, M. A., Barak, S., Mohamed, M., & Penn, A. A. (2021). Placental pathology and intraventricular hemorrhage in preterm and small for gestational age infants. Journal of Perinatology, 41(4), 843–849. https://doi.org/10.1038/s41372-021-00954-6
- Robinson, S. (2012). Neonatal posthemorrhagic hydrocephalus from prematurity: Pathophysiology and current treatment concepts: A review. Journal of Neurosurgery. Pediatrics, 9(3), 10.3171/2011.12.PEDS11136. https://doi.org/10.3171/2011.12.PEDS11136
- Starr, R., De Jesus, O., Shah, S. D., & Borger, J. (2024). Periventricular and Intraventricular Hemorrhage. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK538310/
- Villamor-Martinez, E., Fumagalli, M., Mohammed Rahim, O., Passera, S., Cavallaro, G., Degraeuwe, P., Mosca, F., & Villamor, E. (2018). Chorioamnionitis Is a Risk Factor for Intraventricular Hemorrhage in Preterm Infants: A Systematic Review and Meta-Analysis. Frontiers in Physiology, 9, 1253. https://doi.org/10.3389/fphys.2018.01253

Additional Research
Effect of Opioid administration in frequency distribution and phase coupling in various human brain regions- Opioids and lntracranial EEG (iEEG) study
PI: Mark Witcher, M.D., Ph.D. & Aashit Shah, M. D,
The study proposes to investigate effects of opioids on intracranial EEG (iEEG) from various brain regions including one implicated in targets of opioid effects and addiction. The study leverages the data already collected in individuals with intractable epilepsy undergoing depth electrode placement and iEEG monitoring for detennination of seizure focus as part of the Phase II evaluation for epilepsy surgery. They routinely receive opioid analgesics as part of postoperative care while iEEG is being recorded. We propose to study effects of opioids by studying segments of iEEG before and following opioid administration and also study difference between individuals with or without prior history of substance use disorder (Nicotine, Alcohol, Opioid, Cocaine, etc.)
Additional Information
For individuals with medically intractable focal epilepsy that require iEEG monitoring to define the epileptogenic zone, we routinely implant depth electrodes (a small flexible plastic tube of 1.3 mm diameter with embedded small platinum recording electrode and thin wires) into various brain regions under stereotactic guidance (Shah et al., 2017). This is followed by continuous recording of iEEG using these electrodes for several days (on an average 7 days) to capture seizures (also known as Phase II evaluation). We usually implant depth electrodes in following brain regions; hippocampus, amygdala, temporal neocortex, medial and lateral frontal cortex, cingulate, orbitofrontal cortex as well as other areas such as parietal lobe, insula, occipital lobe as dictated by the clinical scenario/needs. Previous research in human and animal models indicates that many of these areas are also implicated as target sites for opioid and analgesic effects (Kjaer et al., 2017; Reakkamnuan et al., 2017; Fadale et al., 2008; Wass et al., 2001; Ross et., 2001). It is also shown that the effects of opioids differ between the agents and also amongst various brain region. For example, it has been established in patients with mesial temporal lobe epilepsy, that: remifentanil, a short acting opioid can increase neuronal spike count by as much as 80% in the temporal lobe while suppressing activity in other regions (Kjaer et al., 2017). Similarly, acute administration of remifentanil was found to increase single or repetitive spikes in epileptogenic zones while decreasing spike count in non-epileptifonn areas (Wass et al., 2001). A separate opioid, alfentanil was also shown to selectively increase spike frequency in epileptic regions of the human brain (Ross et al., 2001) again demonstrating region-specific effects. Developing this technique can certainly be revolutionary in studying the effects of not just opioids, but other neuroactive compounds/medications at very high spatial resolution and help us understand fundamental effects of these agents at the neuronal level.
Intracranial electrode data allows for physiological detail not afforded by other methodologies in human studies
Neural activity patterns related to drug addiction have traditionally been investigated in humans using non-invasive technologies like MEG, EEG, PET, and fMRI (Matsuura et al. 2008; Quaedflieg et al. 2014; Motlagh et al. 2016; Polunina et al. 2004; Dossantos et al. 2018; Watson et al. 2014). These methods are informative about global dynamics but do not provide the physiological detail available in animal studies. Intracranial electrode data allows the observation of neurophysiological data at a finer spatial scale, on par with animal studies; single unit activity and local field potentials can be detected. We will use intracranial electrode data from human patients to dissect the neural dynamics related to opioid addiction, which have not been previously explored. The level of physiological detail offered by intracranial recordings will allow us to identify the key neural circuits underlying opioid addiction; this would be difficult to do using other methodologies.
1. Shah AK, Mittal S. Invasive electroencephalography monitoring: Indications and presurgical planning. Ann Indian Acad Neurol 2014: 17(5):89-9.
2. Kjaer TW, Hogenhaven H, Lee AP, Madsen FF, Jespersen B, Brennum J, Derm L, Moltke FB. Pharmacodynamics of remifentanil. Induced intracranial spike activity in mesial temporal lobe epilepsy. Epilepsy Res. 2017 Ju/;133:41-45.
3. Reakkamnuan C, Cheaha D, Kumarnsit E. Nucleus accumbens local field potential power spectrums, phaseamplitude couplings and coherences following m01phine treatment.Acta Neurobiol Exp (Wars). 2017;77(3):214- 224.
4. Fodale V, Schifilliti D, Pratico C, Santamaria LB. Remifentanil and the brain. Acta Anaesthesia! Scand. 2008 Mar;52(3):319-26.
5. Wass CT, Grady RE, Fessler AJ, Cascino GD, Lozada L, Bechtle PS, Marsh WR, Sharbrough FW, Schroeder DR. Epilepsia. The effects of remifentanil on epileptiform discharges during intraoperative electrocorticography in patients undergoing epilepsy surgery. Epilepsia. 2001 Oct;42(10): 1340-4.
6. Ross J(J), Kearse LA Jr, Barlow MK, Houghton KJ, Cosgrove GR. Alfentanil-induced epileptiform activity: a simultaneous surface and depth electroencephalographic study in complex partial epilepsy. Epilepsia. 2001 Feb:42(2):220-5.
7. Matsuura N, Shibukawa Y, Kato M, Jchinohe T, Suzuki T, Kaneko Y. Ketamine, not fentanyl, suppresses painrelated magnetic fields associated with trigeminally innervated area following CO2 laser stimulation. Neurosci Res. 2008 Oct;62(2):105-11.
8. Quaedjlieg CW, Milnte S, Ka/so E, Sambeth A. Effects of remifentanil on processing of auditory stimuli: a combined MEG/EEG study. J Psychopharmacol. 2014Jan;28(1):39-48.
9. Motlagh F, Ibrahim F, Menke JM, Rashid R, Seghatoleslam T, Habif H Neuroelectrophysiological approaches in heroin addiction research: A review of literatures. J Neurosci Res. 2016 Apr;94(4):297-309.
10. Polunina AG, Davydov DM EEG spectral power and mean frequencies in early heroin abstinence. Prog Neuropsychopharmacol Biol Psychiatry. 2004 Jan;28(1):73-82. PubMed PMJD: 14687860.
11. DosSantos MF, Oliveira AT, Ferreira NR, Carvalho ACP, Rosado de Castro PH The Contribution of Endogenous Modulatory Systems to TMS- and tDCS-Induced Analgesia: Evidence from PET Studies. Pain Res Manag. 2018 Nov 13;2018: 2368386.
12. Watson BJ, Taylor LG, Reid AG, Wilson SJ, Stokes PR. Brooks DJ, Myers JF, Turkheimer FE, Nutt DJ, LingfordHughes AR Investigating expectation and reward in human opioid addiction with [(11) C}raclopride PET Addict Biol. 2014 Nov:19(6):1032-40
____________
Efficacy of Percutaneous Biopsies for Vertebral Osteomyelitis and Discitis
PI: Biraj Patel, M.D
Vertebral osteomyelitis is an infection of the bones that comprise the vertebral (spinal) column, and discitis is an infection of the spinal disc spaces between the vertebral bones (1,2). Vertebral osteomyelitis represents an estimated ~3-5% of all cases of osteomyelitis and has an increasing incidence rate in the United States within the last few decades (3). Symptoms may be non-specific and can initially present with back pain but can progress into more severe clinical sequelae for patients or death (1,2).
This type of infection can come from many environmental or nosocomial sources including trauma to the spine, post-surgical complications, or hematologic spread (through the blood) (1-4). Current literature suggests that Staphylococcus aureus may represent the most common pathogen responsible for vertebral osteomyelitis infection, however, other strains of microorganisms may be the culprit that causes this condition (1-4). Moreover, there is no current data source that indicates which opportunistic microorganisms may be most prevalent in causing vertebral osteomyelitis infections within our region or health system. This information would be beneficial to clinical practice by providing more targeted management for the source of the infection or how we may prophylactically prevent infections from occurring.
The aims of this study are to:
1. Investigate the positivity rate of vertebral osteomyelitis/discitis cases at Carilion Clinic based on percutaneous biopsy results.
2. Identify leading microbiological sources of infection to mitigate nosocomial- or environmental-acquired infection of the vertebra.
This study will be a retrospective chart review with a timeframe implemented from 1 Jan 2013 through 10 Nov 2023. Retrospective data will be collected from medical records of patients who have had a diagnosis of either vertebral osteomyelitis or discitis and received a biopsy of these infected vertebral structures.
This study will address the positivity rates of percutaneous biopsy results among our patient population. Data from this study will aid in guiding the clinical practice of infectious disease, neurosurgical, and orthopedic specialists regarding the utility of percutaneous biopsies for patients with vertebral osteomyelitis and discitis to develop an optimal treatment plan and potentially decrease overutilization.
____________
Central Nervous System Tissue, Blood and Cerebrospinal Fluid Repository
PI: Mark Witcher, M.D., Ph.D.
A repository is a collection of specimen or data that is saved in a secure location for future use. The aim of the present study is to develop a central neural tissue, blood, and cerebrospinal fluid (CSF) repository to be used in future research studies, and/or educational training experiences at Carilion Clinic, Virginia Tech, or other research institutions to study the underlying mechanisms of any neurosurgical involved disease or disorder. Participants voluntarily consent before their surgery to donate their specimen (blood, tissue, CSF) which would otherwise already be collected but just be discarded as waste. The goal of this study is to buildup supply of tissues of interests to be used in other studies looking to identify new treatment targets for neurosurgical related conditions and improve patient outcomes.
Previous Research
Academic Year 2024–25
Bhutada AS, Adhikari S, Cuoco JA, Patel VM, Olasunkanmi AL. Development and validation of a predictive nomogram for patient with myxopapillary ependymoma: A Surveillance, Epidemiology, and End Results retrospective cohort analysis. Global Spine Journal. 2025;15(4):1905-1913.
Cuoco JA, Witcher MR. In Reply: Spinal intradural arachnoid cysts in adults: An institutional experience and literature review. Neurosurgery. 2024;95(6):e180.
Ladner LR, Adhikari S, Bhutada AS, Cuoco JA, Patel VM, Entwistle JJ, Rogers CM, Marvin EA. Traditional prostate cancer risk assessment scales do not predict outcomes from brain metastases: A population-based predictive nomogram. Cancers. 2024;16(17):3029.
Thibault D, Ravina K, Cuoco JA, Adhikari S, Stump MS, Rogers CM. Racemose neurocysticercosis of the basal arachnoid cisterns: illustrative case. Journal of Neurosurgery: Case Lessons. 2024;8(18):CASE24489.
Cuoco JA, Williams S, Klein BJ, Borowicz VM, Ho H, Stump MS, Rogers CM. Astroblastoma with a novel YAP1:BEND2fusion: A case report. Journal of Pediatric Hematology / Oncology. 2024;46(5):e313-316.
Bhutada AS, Adhikari S, Cuoco JA, et al. Prognostic Factors and Nomogram for Choroid Plexus Tumors: A Population-Based Retrospective Surveillance, Epidemiology, and End Results Database Analysis. Cancers (Basel). 2024;16(3):610. Published 2024 Jan 31. doi:10.3390/cancers16030610
Bhutada AS, Ravina K, Adhikari S, Dodson N, Darden J, Howes GA. Clinical Characteristics and Post-surgical Outcomes in Patients with ACIS™ -Anterior Cervical Interbody Spacer (ACIS) Placement in Anterior Cervical Discectomy and Fusion Surgery. Journal of Spine Research and Surgery. 7 (2025): 44-49. doi:10.26502/fjsrs0087
Ladner L, Bhutada A, Adhikari S, Cuoco J, Entwistle JJ, Rogers C, Marvin E. (2024) Prognostic indicators for pancreatic metastases to the brain: a population-based retrospective Surveillance, Epidemiology, and End Results database analysis. World Neurosurgery. https://doi.org/10.1016/j.wneu.2023.12.01
Ladner L, Schick T, Adhikari S, Marvin E, Weppner J, Kablinger A. (2024) Association between impulsivity, self-harm, suicidal ideation, and suicide attempts in traumatic brain injury patients. Journal of Neurotrauma. https://doi.org/10.1089/neu.2024.0167
Ladner L, Bhutada A, Adhikari S, Cuoco J, Patel V, Entwistle JJ, Marvin E, Rogers C. (2024) Traditional prostate cancer risk assessment scales do not predict outcomes from brain metastases: a population-based predictive nomogram. Cancers. https://doi.org/10.3390/cancers16173029
Ravina K, Peddamallu R, Zia F, Yim B. Mini Pterional Craniotomy for Clip Ligation of a Large Middle Cerebral Artery Bifurcation Aneurysm by Picket-Fence Technique: 2-Dimensional Operative Video. Oper Neurosurg (Hagerstown). 2025 Jan 6. doi: 10.1227/ons.0000000000001487. Epub ahead of print. PMID: 39760504.
Ammothumkandy A, Corona L, Ravina K, Wolseley V, Nelson J, Atai N, Abedi A, Jimenez N, Armacost M, D'Orazio LM, Zuverza-Chavarria V, Cayce A, McCleary C, Nune G, Kalayjian L, Lee DJ, Lee B, Chow RH, Heck C, Russin JJ, Liu CY, Smith JAD, Bonaguidi MA. Human adult neurogenesis loss corresponds with cognitive decline during epilepsy progression. Cell Stem Cell. 2025 Feb 6;32(2):293-301.e3. doi: 10.1016/j.stem.2024.11.002. Epub 2024 Dec 5. PMID: 39642885.
Atai NA, Ravina K, Sizdahkhani S, Rennert RC, Abedi A, Kress G, Fabris F, Nguyen V, Louie S, Shin L, Asante I, Hawes DA, Rossetto O, Carey J, Russin JJ. Botulinum Toxin Application for Treatment of Graft Vasospasm: A Reverse Translational Study. medRxiv [Preprint]. 2024 Nov 13:2024.11.13.24317189. doi: 10.1101/2024.11.13.24317189. PMID: 39606352; PMCID: PMC11601711.
Thibault D, Ravina K, Cuoco JA, Adhikari S, Stump MS, Rogers CM. Racemose neurocysticercosis of the basal arachnoid cisterns: illustrative case. J Neurosurg Case Lessons. 2024 Oct 28;8(18):CASE24489. doi: 10.3171/CASE24489. PMID: 39467317; PMCID: PMC11525761.
Ravina K, Ladner L, Safransky M, Sconzo D, Wetsel ZP, Wadhwa A, Balagurunath K, Ahrens ML, Binello E. Effects of optimal versus suboptimal median household income on the surgically treated traumatic brain injury population at a level I trauma center in the Boston metropolitan area: a propensity score-matched analysis. J Neurosurg. 2024 Dec 13;142(4):1025-1034. doi: 10.3171/2024.7.JNS2440. PMID: 39671591.
Ravina K, Finch IJ, Patel B, Yim B. Y-Stent Technique Using Dual Comaneci Embolization Assist Devices for Coil Embolization of a Ruptured Wide-Necked Anterior Communicating Artery Aneurysm: A Technical Case Report. Oper Neurosurg (Hagerstown). 2024 Sep 1;27(3):365-369. doi: 10.1227/ons.0000000000001143. PMID: 38578712.
Christopher C. Paiz, Oluwafemi P. Owodunni, Evan N. Courville, Meic Schmidt, Robert Alunday, Christian A. Bowers,Frailty Predicts 30-day mortality following major complications in neurosurgery patients: The risk analysis index has superior discrimination compared to modified frailty index-5 and increasing patient age, World Neurosurgery: X, Volume 23, 2024, 100286, ISSN 2590-1397, https://doi.org/10.1016/j.wnsx.2024.100286.
Covell, M.M., Roy, J.M., Gupta, N. et al. Frailty in intracranial meningioma resection: the risk analysis index demonstrates strong discrimination for predicting non-home discharge and in-hospital mortality. J Neurooncol 169, 85-93 (2024). https://doi.org/10.1007/s11060-024-04703-5
Uzoukwu, Cynthia1; Owodunni, Oluwafemi P.2,3,*; Peter-Okaka, Uchenna4; Courville, Evan N.3,5; Conti, Joseph T.6; Gagliardi, Thomas A.6; Schmidt, Meic H.3,5; Bowers, Christian A.3. Impact of frailty on all-cause mortality in older neurosurgical patients with prolonged hospitalization: a retrospective observational study. Aging Advances 1(2):p 69-77, December 2024. | DOI: 10.4103/AGINGADV.AGINGADV-D-24-00016
Bowers, Christian A. MD*,†; Covell, Michael M. BA*,‡; Levy, Elad I. MD, MBA§,?,,**,††; Segura, Aaron C. MSc*,‡‡; Varela, Samantha MD§§; Soliman, Mohamed A. R. MD, MSc, PhD§,??; Mullin, Jeffrey P. MD§,,**; Courville, Evan MD; Quiceno, Esteban MD§,?; Roy, Joanna MD***; Moisi, Marc MD*,†; Delashaw, Johnny MD*,†; Hall, Daniel E. MD, MDiv, MHSc†††,‡‡‡,§§§; Chambless, Lola B. MD???; Piccirillo, Sara G. M. PhD,****; Kogan, Michael MD, PhD*. Arms Race Control Score Standardizes Residency Applicant Publication Assessment. Neurosurgery ():10.1227/neu.0000000000003372, February 17, 2025. | DOI: 10.1227/neu.0000000000003372
Kearns KN, Kurker KP, Marino AC, Zhao P, Ramanathan P, Shaffrey ME, Jane JA Jr, Park MS. Academic Neurosurgery Gender and Authorship Trends in the United States. Neurosurgery. 2025 Jun 1;96(6):1200-1205. doi: 10.1227/neu.0000000000003252. Epub 2024 Oct 28. PMID: 39465950.
Dumot C, Mantziaris G, Dayawansa S, Brantley C, Lee CC, Yang HC, Peker S, Samanci Y, Mathieu D, Tourigny JN, Martinez Moreno N, Martinez Alvarez R, Chytka T, Liscak R, Speckter H, Lazo E, Brito A, Picozzi P, Franzini A, Alzate J, Mashiach E, Bernstein K, Kondziolka D, Tripathi M, Bowden GN, Warnick RE, Sheehan D, Sheehan K, Fuentes A, Jane JA, Vance ML, Sheehan JP. Risk of new tumor, carotid stenosis, and stroke after stereotactic radiosurgery for pituitary tumor: A multicenter study of 2254 patients with imaging follow-up. Neuro Oncol. 2024 Dec 5;26(12):2328-2338. doi: 10.1093/neuonc/noae133. PMID: 39028740; PMCID: PMC11630564.
Academic Year 2023–24
Marlow C, Cuoco JA, Hoggarth AR, Stump MS, Apfel LS, Rogers CM. Pediatric diffuse hemispheric glioma H3 G34-mutant with gains of the BRAF locus: An illustrative case. Rare Tumors. 2023;15:20363613231168704. Published 2023 Apr 7. doi:10.1177/20363613231168704
Hoggarth AR, Muthukumar S, Thomas SM, Crowley J, Kiser J, Witcher MR. Clinical Theranostics in Recurrent Gliomas: A Review. Cancers (Basel). 2024;16(9):1715. Published 2024 Apr 28. doi:10.3390/cancers16091715
Bhutada AS, Hoggarth AR, Adhikari S, et al. Prognostic factors of survival for grade III solitary fibrous tumor/hemangiopericytoma: a population-based retrospective Surveillance, Epidemiology, and End Results database analysis. Oncology. Published online December 30, 2023. doi:10.1159/000535823
Bhutada AS, Adhikari S, Cuoco J, In A, Entwistle J, Witcher MR. Co-occurrence of dural arteriovenous fistula and meningioma: A rare case and systematic review. World Neurosurg X. 2023 May 11;19:100217. PMID: 37235061; PMCID: PMC10206830.
Cuoco JA, Guilliams EL, Adhikari S, Rogers CM, Marvin EA, Patel BM, Entwistle JJ. Systemic Immune-Inflammation Index Predicts Acute Symptomatic Hydrocephalus After Spontaneous Nonaneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2023 May;173:e378-e390. PMID: 36804432.
Adhikari S, Bhutada AS, Ladner L, Cuoco JA, Entwistle JJ, Marvin EA, Rogers CM. Prognostic Indicators for H3K27M-Mutant Diffuse Midline Glioma: A Population-Based Retrospective Surveillance, Epidemiology, and End Results Database Analysis. World Neurosurg. 2023 Oct;178:e113-e121. PMID: 37423332.
Bhutada AS, Adhikari S, Cuoco JA, In A, Rogers CM, Jane JA Jr, Marvin EA. Prognostic Factors and Nomogram for Choroid Plexus Tumors: A Population-Based Retrospective Surveillance, Epidemiology, and End Results Database Analysis. Cancers (Basel). 2024 Jan 31;16(3):610. PMID: 38339361; PMCID: PMC10854689.
Ladner L, Bhutada AS, Adhikari S, Cuoco JA, Entwistle JJ, Rogers CM, Marvin EA. Prognostic Indicators for Intracranial Metastases from Pancreatic Cancer: A Population-Based Retrospective Surveillance, Epidemiology, and End Results Database Analysis. World Neurosurg. 2024 Feb;182:e666-e674. PMID: 38070735.
Bhutada AS, Adhikari S, Cuoco JA, Rogers CM, Marvin EA. Survival Benefit from Multimodal Treatment for Patients with Atypical Teratoid Rhabdoid Tumor in a Surveillance, Epidemiology, and End Results Database Analysis. Oncology. 2024;102(2):183-194. PMID: 37634491.
Ravina K., Patel B., Yim B. Y-Stent Technique Utilizing Dual Comaneci Embolization Assist Devices for Coil Embolization of a Ruptured Wide-Necked Anterior Communicating Artery Aneurysm: A Technical Case Report. Oper Neurosurg (Hagerstown). 2024 Apr 4. doi: 10.1227/ons.0000000000001143. Epub ahead of print. PMID: 38578712.
Ravina K., Patel B., Yim B. Spontaneous development, and involution of a de novo pseudoaneurysm at the superficial temporal artery - middle cerebral artery bypass anastomotic site in a patient with Moyamoya disease: illustrative case. J Neurosurg Case Lessons. 2024 Jan 22;7(4):CASE23665. doi: 10.3171/CASE23665. PMID: 38252937; PMCID: PMC10805593.
Ravina K., Adhikari S., Bhutada A., Marvin E. Survival Determinants of Patients with Uncertain Behavior Pituitary Tumors: a Surveillance, Epidemiology, and End Results (SEER) Database Study. Brain Disorders 13(1):100108. DOI: 10.1016/j.dscb.2023.100108.
Marino AC, Farzad F, Jane JA Jr.: Chiari I Malformation and Sleep-Disordered Breathing. Neurosurg Clin N Am. 2023 Jan;34(1):35-41. doi: 10.1016/j.nec.2022.08.005. PMID: 36424062
Ironside N, Chen CJ, Xu Z, Schlesinger D, Lee Vance M, Hong GK, Jane JA Jr, Patel S, Bindal SK, Niranjan A, Lunsford LD, Liscak R, Chytka T, Jezkova J, Saifi O, Trifiletti DM, Berger A, Alzate J, Bernstein K, Kondziolka D, Speckter H, Hernandez W, Lazo E, Peker S, Samanci Y, Zacharia BE, Mau C, Wegner RE, Shepard MJ, Mathieu D, Maillet M, Sheehan JP.: Effects of Neuroantomic Structural Distances on Pituitary Function After Stereotactic Radiosurgery: A Multicenter Study. Neurosurgery. 2023 May 1;92(5):1035-1042. doi: 10.1227/neu.0000000000002347. Epub 2023 Jan 17. PMID: 36700741
Kearns KN, Rabinovich EP, Shabo L, Shaffrey ME, Jane JA Jr, Park MS.: Composition and Gender Distribution of Editorial Boards for Top Neurosurgical Journals. World Neurosurg. 2023 Aug;176:189-198. doi: 10.1016/j.wneu.2023.05.009. Epub 2023 May 9. PMID: 37169075
Drexler R, Rotermund R, Smith TR, Kilgallon JL, Honegger J, Nasi-Kordhishti I, Gardner PA, Gersey ZC, Abdallah HM, Jane JA, Marino AC, Knappe UJ, Uksul N, Rzaev JA, Galushko EV, Gormolysova EV, Bervitskiy AV, Schroeder HWS, Eördögh M, Losa M, Mortini P, Gerlach R, Azab M, Budohoski KP, Rennert RC, Karsy M, Couldwell WT, Antunes ACM, Westphal M, Ricklefs FL, Flitsch J.: Defining benchmark outcomes for transsphenoidal surgery of pituitary adenomas: a multicenter analysis. Eur J Endocrinol. 2023 Sep 1;189(3):379-386. doi: 10.1093/ejendo/lvad124. PMID: 37668325
Findlay MC, Drexler R, Azab M, Karbe A, Rotermund R, Ricklefs FL, Flitsch J, Smith TR, Kilgallon JL, Honegger J, Nasi-Kordhishti I, Gardner PA, Gersey ZC, Abdallah HM, Jane JA Jr, Marino AC, Knappe UJ, Uksul N, Rzaev JA, Bervitskiy AV, Schroeder HWS, Eördögh M, Losa M, Mortini P, Gerlach R, Antunes ACM, Couldwell WT, Budohoski KP, Rennert RC, Karsy M.: Crooke Cell Adenoma Confers Poorer Endocrinological Outcomes Compared with Corticotroph Adenoma: Results of a Multicenter, International Analysis. World Neurosurg. 2023 Dec;180:e376-e391. doi: 10.1016/j.wneu.2023.09.076. Epub 2023 Sep 25. PMID: 37757948
Findlay MC, Drexler R, Khan M, Cole KL, Karbe A, Rotermund R, Ricklefs FL, Flitsch J, Smith TR, Kilgallon JL, Honegger J, Nasi-Kordhishti I, Gardner PA, Gersey ZC, Abdallah HM, Jane JA Jr, Marino AC, Knappe UJ, Uksul N, Rzaev JA, Galushko EV, Gormolysova EV, Bervitskiy AV, Schroeder HWS, Eördögh M, Losa M, Mortini P, Gerlach R, Antunes ACM, Couldwell WT, Budohoski KP, Rennert RC, Azab M, Karsy M: A Multicenter, Propensity Score-Matched Assessment of Endoscopic Versus Microscopic Approaches in the Management of Pituitary Adenomas. Neurosurgery. 2023 Oct 1;93(4):794-801. doi: 10.1227/neu.0000000000002497. Epub 2023 Apr 14. PMID: 37057921
Catalino MP, Moore DT, Ironside N, Munoz AR, Coley J, Jonas R, Kearns K, Min L, Vance ML, Jane JA Jr, Laws ER Jr: Post-operative serum cortisol and Cushing Disease recurrence in patients with corticotroph adenomas. J Clin Endocr Metab, 2023 Dec 108(12): 3287-3294. 2023; https://doi.org/10.1210/clinem/dgad347
McClure JJ, Chatrath A, Robison TR, Jane JA Jr: Conditioned recurrence-free survival following gross-total resection of nonfunctioning pituitary adenoma: a single-surgeon, single-center retrospective study. J Neurosurg Published online December 8, 2023; DOI: 10.3171/2023.10.JNS23754
Findlay MC, Sabahi M, Azab M, Drexler R, Rotermund R, Ricklefs FL, Flitsch J, Smith TR, Kilgallon JL, Honegger J, Nasi-Kordhishti I, Gardner PA, Gersey ZC, Abdallah HM, Jane JA, Knappe UJ, Uksul N, Schroder HWS, Eördögh M, Losa M, Mortini P, Gerlach R, Antunes ACM, Couldwell WT, Budohoski KP, Rennert RC, Karsy M: The role of surgical management for prolactin-secreting tumors in the era of dopaminergic agonists: An international multicenter report. Clin Neurol Neurosurg. 2024 Jan;236:108079. doi: 10.1016/j.clineuro.2023.108079. Epub 2023 Dec 10. PMID: 38091700
Fuentes AM, Yun JJ, Jane JA Jr: Nontraumatic symptomatic de novo arachnoid cyst in an adolescent patient treated with cystoperitoneal shunting: illustrative case. J Neurosurg Case Lessons 7(5): CASE23584, 2024. doi.org/10.3171/CASE23584.
Mamelek AN, Little AS, Gardner PA, Almeida JP, Recinos P, Soni P, Kshettry VR, Jane JA Jr, Barkhoudarian G, Kelly DF, Dodd R, Mukherjee D, Gersey ZC, Fukuhara N, Nishioka H, Kim EH, Litre CF, Sina E, Mazer MW, Cui Y, Bonert V: A prosective, multicenter, observational study of surgical vs nonsurgical management for pituitary apoplexy. J Clin Endocr Metab, 2024 Feb 109(2): e711-e725, https://doi.org/10.1210/clinem/dgad541
Fuentes AM, Jane JA Jr: Spontaneous regression of an interhemispheric arachnoid cyst: illustrative case. Childs Nerv Syst. 2024 May 18. doi: 10.1007/s00381-024-06464-y. Online ahead of print.
Academic Year 2022–23
Bhutada AS, Adhikari S, Cuoco JA, Entwistle JJ, Witcher MR. Co-occurrence of dural arteriovenous fistula and meningioma: A rare case and systematic review. World Neurosurgery: X. 2023;19:100217.
Cuoco JA, Guilliams EL, Adhikari S, Rogers CM, Marvin EA, Patel BM, Entwistle JJ. Systemic immune-inflammation index predicts acute symptomatic hydrocephalus after spontaneous non-aneurysmal subarachnoid hemorrhage. World Neurosurgery. 2023;173:e378-390.
Cuoco JA, Guilliams EL, Marvin EA, Patel BM, Entwistle JJ. Perimesencephalic subarachnoid hemorrhage has a unique peripheral blood leukocyte profile compared to aneurysmal subarachnoid hemorrhage. World Neurosurgery. 2022;163:e471-481.
Cuoco JA, Muthukumar S, Rogers CM, Entwistle JJ, Patel V, Olasunkanmi AL, Witcher MR. Spinal intradural arachnoid cysts in adults: An institutional experience and literature review. Neurosurgery. 2023;92(3):450-463.
Cuoco JA, Strohman A, Stopa BM, Stump MS, Entwistle JJ, Witcher MR, Olasunkanmi AL. Supratentorial cortical ependymomas: A systematic literature review and case illustration. Rare Tumors. 2022;14:1-15.
In A, Stopa BM, Cuoco JA, Olasunkanmi AL, Entwistle JJ. Depressed skull fracture compressing eloquent cortex causing focal neurologic deficits. Brain Injury. 2023;37(4):352-355.
Langman M, Stopa BM, Cuoco JA, Patel V, Rogers CM, Marvin EA. Natural history of traumatic encephaloceles: A systematic literature review. Journal of Craniofacial Surgery. 2023;34(1):120-125.
Marlow C, Cuoco JA, Hoggarth AR, Stump MS, Apfel LS, Rogers CM. Pediatric diffuse hemispheric glioma H3-G34-mutant with gains of the BRAF locus: An illustrative case. Rare Tumors. 2023;15:1-7.
Marlow C, Cuoco JA, Ravina K, Sloboda C, Entwistle JJ. Endovascular treatment of a ruptured pure arterial malformation and associated dysplastic middle cerebral artery dissecting aneurysm: illustrative case. Journal of Neurosurgery: Case Lessons. 2023;5(21):CASE23150.
Ravina K, Windermere SA, Zhao Q, Lerner A, Dyer M, Upadhyay U, Jha RT. Primary intracranial extraosseous Ewing’s sarcoma of the skull in an elderly adult: illustrative case. Journal of Neurosurgery: Case Lessons. 2022;4(16):CASE22214.
Ravina K, Yang N, Brocoum S, Pasco-Anderson J, Walker RL, Khan M, Cabodi M, Holsapple J. Conical drill bit for optimized external ventricular drain placement: A proof-of-concept study. Accepted / In-Press in Journal of Neurosurgery
Stopa BM, Cuoco JA, Stump MS, Rogers CM. Supratentorial neurenteric cysts: Systematic literature review and case report. World Neurosurgery. 2022;164:8-24.
Wolfswinkel EM, Ravina K, Rennert RC, Landau M, Strickland BA, Chun A, Wlodarczyk JR, Abedi A, Carey JN, Russin JJ. Cerebral bypass using the descending branch of the lateral circumflex femoral artery: A case series. Operative Neurosurgery. 2022;22(6):364-372.
Academic Year 2021–22
Cuoco JA, Guilliams EL, Entwistle JJ, Patel BM, Marvin EA. Initial intracranial pressure is an independent predictor of unfavorable functional outcomes after aneurysmal subarachnoid hemorrhage. Journal of Clinical Neuroscience. 2021;94:152-158.
Cuoco JA, Guilliams EL, Klein BJ, Benko MJ, Darden JA, Olasunkanmi AL, Witcher MR, Rogers CM, Marvin EA, Patel BM, Entwistle JJ. Neutrophil count on admission predicts acute symptomatic hydrocephalus after aneurysmal subarachnoid hemorrhage. World Neurosurgery. 2021;156:e338-344.
Cuoco JA, Guilliams EL, Klein BJ, Witcher MR, Marvin EA, Patel BM, Entwistle JJ. Monocyte count on admission is predictive of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. Frontiers in Surgery. 2022;9:879050.
Cuoco JA, Klein BJ, Lebel DP, Faulhaber JR, Apfel LS, Witcher MR. Successful treatment of a Balamuthia mandrillaris cerebral abscess in a pediatric patient with complete surgical resection and antimicrobial therapy: Case report and literature review. The Pediatric Infectious Disease Journal. 2022;41(2):e54-57.
Cuoco JA, Rogers CM. In Reply to the Letter to the Editor Regarding: “Postexercise Death Due to Hemorrhagic Colloid Cyst of Third Ventricle: Case Report and Literature”. World Neurosurgery. 2021;153:153.
Stopa BM, Cuoco JA, Adhikari S, Grider DJ, Rogers CM, Marvin EA. Iatrogenic leptomeningeal carcinomatosis status post craniotomy for resection of metastatic serous ovarian carcinoma: Systematic literature review and case report. Frontiers in Surgery. 2022;9:850050.
Academic Year 2020–21
Cuoco JA, Benko MJ, Klein BJ, Keyes DC, Patel BM, Witcher MR. Idiopathic fourth ventricular outlet obstruction misdiagnosed as normal pressure hydrocephalus: A cautionary case. Surgical Neurology International. 2020;11:305.
Cuoco JA, Guilliams EL, Apfel LS, Marvin EA, Patel BM. Incidental pediatric high-flow non-Galenic giant pial arteriovenous fistula. Neuropediatrics. 2021;52(1):65-68.
Cuoco JA, Guilliams EL, Klein BJ, Jarrett RW, Entwistle JJ, Marvin EA. Atypical presentation of Langerhans cell histiocytosis of the skull. JAMA Otolaryngology - Head & Neck Surgery. 2020;146(10):975-977.
Cuoco JA, Guilliams EL, Rogers CM, Entwistle JJ, Olasunkanmi AL. Multicentric benign demyelinating pseudotumor: The great masquerade. The Neurohospitalist. 2020;10(4):326-328.
Cuoco JA, Guilliams EL, Rogers CM, Patel BM, Marvin EA. Recurrent cerebral vasospasm and delayed cerebral ischemia weeks subsequent to elective clipping of an unruptured middle cerebral artery aneurysm. World Neurosurgery. 2020;141:52-58.
Cuoco JA, Klein BJ, Kar A, Gosnell HL, Guilliams EL, Benko MJ, Apfel LS, Entwistle JJ, Marvin EA, Witcher MR. Factors affecting R01 grant funding among academic neurosurgeons over the past decade. Annals of Medicine and Surgery. 2020;55:260-264.
Cuoco JA, Kortz MW, Benko MJ, Jarrett RW, Rogers CM, Witcher MR, Marvin EA. Pineal gland metastasis from poorly differentiated carcinoma of unknown primary origin. Frontiers in Endocrinology. 2020;11:597773.
Cuoco JA, Kortz MW, McCray E, Guilliams EL, Busch CM, Rogers CM, Mittal S. Case report: Metastatic bronchopulmonary carcinoid tumor to the pineal gland. Frontiers in Endocrinology. 2021;12:623756.
Cuoco JA, Rogers CM, Mittal S. The oncolytic Newcastle disease virus as an effective immunotherapeutic strategy against glioblastoma. Neurosurgical Focus. 2021;50(2):E8.
Klein BJ, Cuoco JA, Rogers CM, Entwistle JJ, Marvin EA, Patel BM. Delayed cerebral ischemia causing cortical blindness due to repeat cocaine use weeks subsequent to aneurysmal subarachnoid hemorrhage. Radiology Case Reports. 2020;15(9):1455-1459.
Marvin EA, Furrow K, Kar A, Cuoco JA. Response of pembrolizumab alone for non-small cell lung cancer with brain metastasis: A case report and literature review. Frontiers in Oncology. 2020;10:577159.
Academic Year 2019–20
Benko MJ, Abdulla SG, Cuoco JA, Dhiman N, Klein BJ, Guilliams EL, Marvin EA, Howes GA, Collier BR, Hamill ME. Short and long-term geriatric mortality after acute traumatic subdural hemorrhage. World Neurosurgery. 2019;130:350-355.
Cuoco JA, Guilliams EL, Klein BJ, Malaty GR, Witcher MR, Entwistle JJ. N-butyl cyanoacrylate embolization of a traumatic pseudoaneurysm and arteriovenous fistula of the middle meningeal artery. Radiology Case Reports. 2020;15(4):321-325.
Cuoco JA, Klein BJ, Busch CM, Gosnell HL, Kar A, Marvin EA, Apfel LS. Neurosurgical management of lateral meningocele syndrome: A clinical update for the pediatric neurosurgeon. Pediatric Neurosurgery. 2020;55(1):2-11.
Cuoco JA, Klein BJ, Busch CM, Guilliams EL, Olasunkanmi AL, Entwistle JJ. Corticosteroid-induced regression of glioblastoma: A radiographic conundrum. Frontiers in Oncology. 2019;9:1288.
Cuoco JA, Rogers CM, Busch CM, Apfel LS, Entwistle JJ, Marvin EA. Intracranial squamous cell carcinoma arising from an epidermoid cyst remnant four decades after partial resection. Frontiers in Oncology. 2019;9:694.
Dominguez L, Saway B, Benko MJ, Guilliams E, Marvin EA, Entwistle JJ. Ruptured Distal Superior Cerebellar Artery Aneurysm After Gamma Knife Radiosurgery for Trigeminal Neuralgia: A Case Report and Review of the Literature. World Neurosurgery. 2020;135:2-6.
Godbe KN, Saway BF, Guilliams EL, Entwistle JJ, Jarrett RW Jr. Spontaneous necrotizing granuloma of the cerebellum: a case report. BMC Neurology. 2020;20:230.
Kar A, Guilliams EL, Cuoco JA, Marvin EA. Rapidly fatal encephalitis associated with atypical lymphoid proliferations of the basal ganglia subsequent to aneurysmal subarachnoid hemorrhage. Clinics and Practice. 2019;9:1187.
Rogers CM, Palmerton H, Saway B, Tomlinson D, Simonds G. Effect of Various OR Noise on Fine Motor Skills, Cognition, and Mood. Surgery Research and Practice. 2019;5372174.
Rogers CM, Saway B, Busch CM, Simonds GR. The Effects of 24-Hour Neurosurgical Call on Fine Motor Dexterity, Cognition, and Mood. Cureus. 2019;11(9):e5687.
Roshandel AK, Busch CM, Van Mullekom JV, Cuoco JA, Rogers CM, Apfel LS, Marvin EA, Sontheimer HW, Umans RA. The predictive capability of immunohistochemistry and DNA sequencing for determining TP53 functional mutation status: A comparative study of 41 glioblastoma patients. Oncotarget. 2019;10(58):6204-6218.
Academic Year 2018–19
Benko MJ, Danison AP, Marvin EA, Saway BF. Distal Cauda equina syndrome: A case report of lumbosacral disc pathology and review of literature. Surgical Neurology International. 2019;10:84.
Busch CM, Prickett JT, Stein R, Cuoco JA, Marvin EA, Witcher MR. Meckel’s cave epidermoid cyst presenting as multiple cranial nerve deficits due to indirect tumoral compression of the cavernous sinus: A case report and literature review. World Neurosurgery. 2019;121:88-94.
Cuoco JA, Benko MJ, Busch CM, Rogers CM, Prickett JT, Marvin EA. Vaccine-based immunotherapeutics for the treatment of glioblastoma: Advances, challenges, and future perspectives. World Neurosurgery. 2018. 120:302-315.
Cuoco JA, Busch CM, Klein BJ, Benko MJ, Stein R, Nicholson AD, Marvin EA. ACTA2 cerebral arteriopathy: Not just a puff of smoke. Cerebrovascular Diseases. 2018;46(3-4):159-169.
Cuoco JA, Busch CM, Rogers, CM, Guilliams EL, Klein BJ, Howes GA, Marvin EA. Quantitative description of osteopathic physician authorship in prominent neurosurgery journals since 1944 - Coming of age? Cureus. 2018;10(8):e3124.
Cuoco JA, Rogers CM, Busch CM, Benko MJ, Apfel LS, Elias Z. Postexercise death due to hemorrhagic colloid cyst of the third ventricle: Case report and literature review. World Neurosurgery. 2019;123:351-356.
Cuoco JA. Restoration of motor function after operative reconstruction of the acutely transected spinal cord in the canine model. Surgery. 2019;165(2):486-496.
Rogers CM, Busch CM, Cuoco JA, Elias Z, Simonds GR. Economic impact of hospitalization past maximal neurosurgical inpatient benefit. Cureus. 2018;10(11): e3567.
Rogers CM, Simonds G, Mayo DA, Linskey ME, Phelps J. Commentary: Addressing Concerns Regarding the Evolving “Disruptive Physician” Label. Neurosurgery. 2019;84(3):E225-E229.
Our Research Team
Research initiatives at Carilion are centered around our patients. Here, participating patients, their care teams and our researchers work together to develop new medicines, innovative devices and advanced procedures that may benefit them—and countless others—in the years to come.
Our researchers are practicing medical professionals from across the nation who have many years of experience in their specialty. Our neurosurgery group includes attending physicians, resident physicians, medical students, nursing personnel, advanced care practitioners and trained research specialists.
Research Specialists
Jordan Darden, PhD
Director of Neurosurgery Research
Jordan is a translational scientist with over 10 years' experience in clinical research. A native of southwest Virginia, she started out in the psychology field studying human behaviors and spent several years in Texas investigating post-traumatic stress disorder in military personnel. After seeing the impact of current standards, she wanted to help understand the underlying basis of behaviors and human disease.
Jordan attended graduate school for a biomedical doctorate degree and studied the translational research spectrum, focusing on preclinical and clinical models. She joined Carilion in April 2019 with the Department of Surgery. Her passion is finding new and better ways to help obtain resources, educate and treat her hometown community.

Nanci Dodson, BS, CHES
Clinical Research Coordinator
Nanci has a B.S. in health promotion and is currently pursuing her master of health sciences. Her interest in research is rooted in curiosity and the desire to find more approaches to care. In 2014, Nanci's grandmother fell while chopping wood and experienced a subdural hematoma. She was then in a coma for nearly a month. Her health was restored thanks to the neurosurgeons who helped save her life, along with her overall health pre-injury. Seeing her grandmother’s recovery firsthand inspired Nanci to learn more about post-traumatic outcomes.
Nanci believes that individualized care helps create a path for scientific discovery. Contributing to those discoveries through clinical trials is one of the many reasons Nanci loves working in research.
Nanci enjoys rock climbing, hiking, and hanging out with her husband and their three adorable pitbulls.

Erica Smith, BS, HCM
Resident Program Manager
Erica was born and raised in upstate New York. After proudly serving in the U.S. Army, she received an associate’s degree in surgical technology and a bachelor’s degree in health care management from Jefferson College of Health Sciences in Roanoke, VA (now Radford University Carilion).
Erica was initially exposed to neurosurgery in the operating room. That wasn’t enough for her, as she was interested in learning what it took to become a neurosurgeon and helping develop future leaders in the field. She transitioned into graduate medical education and has served as the Neurosurgery resident program manager for over five years.
Erica is dedicated to the responsibilities that come along with managing the program. She learns something new every day and sincerely enjoys supporting resident training at Virginia Tech Carilion Neurosurgery.

Jen Preas, RN
Research Navigator

Jessica Ericsson, NP
Research Nurse

Valerie Abbott, PA
Research Specialist
Attendings

John Jane, Jr., MD
Chair, Department of Neurosurgery
Profile Page
John Jane, Jr., is Carilion Clinic's chair of Neurosurgery. He joins us from University of Virginia where he spent years practicing as a pediatric neurosurgeon. Dr. Jane was inspired to pursue Neurosurgery by the example of his father, who taught him to appreciate the value of inquiry, novel ideas, training the next generation and the practice of medicine. His research interests stems from his clinical practice and primarily has involved retrospective analysis of adults and children with pituitary and other tumors arising from the sellar region, as well as craniofacial disorders, Chiari and hydrocephalus.

Mark Witcher MD, PhD
Neurosurgery Resident Program Director
Profile Page
Mark Witcher came to Virginia Tech Carilion after completion of training at Medical College of Georgia, Wake Forest University, and Emory University. During this time, he completed both M.D. and Ph.D. training with a focus in neuroscience, as well as neurosurgery residency and fellowship in Functional Neurosurgery. He was fortunate to train under mentors at each institution focused on translational neuroscience, integrating neurosurgical research into clinical neurosurgical practice. He has had the incredible opportunity to continue this tradition at VTC Neurosurgery. His passion is helping patients with functional neurosurgical issues. He has dedicated many years to studying electrical signaling in the brain to better understand the neurons signaling that cause tremors, seizures, movement disorders, pain, weakness, sensory symptoms and blackouts.

Lisa Apfel, MD
Pediatric Neurosurgeon
Profile Page
Lisa Apfel joins us from New York after completing training at State University of New York at Buffalo. Before her training as a neurosurgeon, Lisa was a Wall Street guru and is a bit of a wonder woman. She also practices homeopathic medicine and holistic approaches, often helping her fellow neurosurgeons with headaches and other common ailments. While she treats a variety of neurological conditions such as cancer and spinal disorders her passion lies in pediatric neurosurgery. She has a variety of research interests that marry her interests in nutrition, pediatrics, and cancers.

Greg Howes, DO
Neurosurgeon
Profile Page
Greg Howes primarily works with industry sponsored clinical trials bringing new medical devices to his patients. His research interests include spinal surgery and pain management.


Eric Marvin, DO
Skull-Based Neurosurgeon
Profile Page
Eric Marvin attended the Lake Erie College of Osteopathic Medicine and completed his residency with us at Carilion Neurosurgery. He went on to complete his fellowship at St. Louis University School of Medicine before rejoining us in 2015. Eric is a skull based neurosurgeon and specializes in complex skull based procedures such as resection of tumors, craniotomiesarotid endarterectomy, craniotomies, awake surgery, neuro-mapping, stereotactic radiosurgery, and emergent head and spine procedures. He is considered to be among the busiest in the OR and often teaches resident trainings for complex skull skills. Eric is known for his compassion, down to earth and bedside manner with his patients. He has a variety of research interests that cover his desire to help improve his patient's lives for the better and furthering understanding and treatment of skull based procedures.

Adeolu Olasunkanmi, MD
Complex Spinal Surgeon
Profile Page
Adeolu “Ade” Olasunkanmi, or Dr. O as he is lovingly called, joined us from the University of Wisconsin-Madison following his training with innovative industry leaders in the treatment and management of complex spine deformities and degenerative disorders of the spine. His initial interest in pursuing a career specializing in spinal neurosurgery is a result of seeing his father’s struggle with post operative care after having a spinal tumor removed. This personal experience lead to a desire and motivation to pursue research in innovative techniques and new management of patients with spinal cord injuries (SCI), spine disorders and complex spine deformities.
An area of particular interest for Ade is spinal cord injury (SCI), and the treatment and management of this lifelong condition. He has been part of multinational trials looking at how to improve patient outcomes for SCI, as well as being actively involved in several new investigative drugs for the treatment of this condition.
Ade is actively involved in the Carilion Innovations vision, working with engineers, technicians, and collaborators in industry and academia to innovate tools, diagnostics, and potential treatments to improve his patients’ quality of life. In his spare time, he loves to travel and is an enthusiastic foodie.

Biraj Patel, MD
Neurointerventional Radiologist
Profile Page
Biraj's research interests over the years have honed into hemorrhagic and ischemic stroke. Stroke is the 5th leading cause of death in the US and primary cause of permanent disability. Over the last decade there have been tremendous improvements in the way we manage stroke due to the hard work of stroke researchers, scientists and physicians. Biraj enjoys being part of this group as we work to make more advancements in treatment of stroke and continue to fight this devastating disease and improve patient outcomes in our region. His work involves hemorrhagic stroke such as cerebral (brain) aneurysms, vascular malformations (AVM, dAVF), chronic subdural hematomas (cSDH). He also has projects involving ischemic stroke including a translational project with his co-PI Dr. Michelle Theus (VT) studying various factors that effect collaterals during acute ischemic stroke. Additionally as the local PI for StrokeNet (Stroke National Capital Area Network for Research - SCANR), he has ongoing access to high level stroke research trials.
Biraj Patel's team is currently preparing to enroll in 2 large national phase III randomized trials: Efficacy and Safety Evaluation of 3K3A-APC in Ischemic Stroke (RHAPSODY-2) and Comparison of Anti-coagulation and Anti-platelet Therapies for Intracranial Vascular Atherostenosis (CAPTIVA).

Vaibhav Patel, MD
Spinal Neurosurgeon
Profile Page
Vaibhav "VP" Patel is a local, growing up in the Radford and Pulaski areas. He rotated in neurosurgery in medical school and fell in love with the field after viewing a hemicraniotomy. He completed his residency at University of Texas Health Science Center in San Antonio and returned to the area to join Carilion Neurosurgery in 2019. He decided to focus his practice into a subspeciality for spine to help the most people possible in his community. Vaibhav is a complex spinal neurosurgeon and specializes in spinal deformities, spinal trauma and complex spinal procedures. He often teaches resident trainings for complex spinal skills and management for these resultant conditions. VP's main interests in research are spinal cord injury and nerve regeneration.


Cara Rogers, DO
Oncology Neurosurgeon
Profile Page
Cara Rogers trained at Virginia Tech Carilion and then completed a neurosurgical oncology fellowship at MD Anderson. Cara returned to Roanoke to join our faculty because she had fallen in love with the community during residency. While she treats a wide range of neurosurgical pathologies, her passion is the treatment of benign and cancerous tumors that affect the brain, skull base, spinal column, spinal cord, and peripheral nerves. Her research interests therefore focus on the advancement of diagnostic and treatment methods for neurological tumors, in particular high grade glial neoplasms. Cara’s primary goal is to provide all of her patients and their loved ones with the most innovative, efficacious, and compassionate care possible.

Brendan Klein, DO
Brendan Klein graduated our Carilion Clinic VTC Neurosurgery Residency Program in June 2023. He went on to complete a year fellowship in Atlanta's Emory University Children's Hospital, the largest pediatric neurosurgery center in the United States. Brendan is welcomed back as our newest pediatric neurosurgeon attending this July 2024!
Residents
The Carilion Clinic Virginia Tech Carilion Neurosurgery Residency Program is an intensive training program with heavy emphasis on surgical decision making, critical medical thinking, neuroscience research, wellness and resilience building, teaching and hands-on surgical experience. Rigorous conference and lecture schedules are backed by extensive textbook and medical journal reading assignments, as well as abundant high-level research opportunities. Please visit our new website for more details on our GME Residency Program at About Us | Neurosurgery Residency | Graduate Medical Education | Carilion Clinic & VTC.
We also have a Resident Instagram page! Come visit us @vtcneurosurgresidency.

Srijan Adhikari, MD
Srijan "Sri" is a resident in Neurosurgery who is in his final year of training. He has a keen interest in open and minimally invasive skull base surgery. Sri's background in Nepal and his passion for global health have motivated Carilion to pursue academic and clinical collaborations in Nepal. Sri is enthusiastic about studying and analyzing large datasets related to brain tumors. When he's not working, Sri enjoys spending quality time with his family, including his two children, and exploring the beautiful natural surroundings of the Blue Ridge area.

Austin Hoggarth, MD
Austin is in his chief year of residency and plans to focus on neuro-oncology related cases as an attending. He is a native of North Dakota and enjoys biking, hiking, eating good food, and watching movies like “No Country for Old Men” and “Midsommer.” Austin claims to be the wine sommelier and best DJ out of all the residents claiming his eclectic music taste is good for diversifying the listening habits of the other doctors. His passion for neurosurgery started when a young family member was diagnosed with a GBM and his drive to help others with similar brain pathology continues through his neurosurgical training. His current research is focused on GBM microenvironments and MRI predictive models in hopes of better understanding and finding a treatment for such a devastating disease.
Kristine Ravina, MD
Kristine is a neurosurgery resident with subspecialty interests in vascular and skull base neurosurgery. She was born in Latvia, a small country by the Baltic Sea in Northern Europe but is a well-traveled world citizen who has lived on both North American coasts as a former resident of Palo Alto, Los Angeles and Boston while pursuing her clinical and research interests. She has completed a two-year post-doctoral fellowship in neuroscience at Stanford University and nearly three years of research work as a research associate at Neurorestoration Center, Keck School of Medicine, University of Southern California with focus on stroke, open vascular, epilepsy surgery and cerebral revascularization. She has also completed a two-year hands-on neurosurgery clinical pre-residency fellowship at Boston Medical Center. She is an inventor and an author of more than 50 peer-reviewed research papers, numerous national and international conference abstracts, invited talks and presentations as well as a successful NIH grant and patent applications. Kristine is inspired by translational research bringing together professionals from different biomedical, engineering, and environmental science backgrounds to create multi-specialty fusion projects that help everyone learn more about the parallels between neurosurgery, neurosciences, and other specialties. She chose Neurosurgery for its multifaceted nature in the crossroads of neuroscience, neurology, surgery, and engineering offering great potential for making a difference and bringing fulfillment of rewarding, hands-on work. Outside of neurosurgery, Dr. Ravina is an outdoors enthusiast passionate about mountaineering, wilderness expeditions, hiking and trail running.

Cole Sloboda, DO
Cole is our resident with a general passion for neurosurgery and is still exploring possible sub-specialties. He became interested in neuroscience in high school and fell in love with neurosurgery during medical school. The opportunity to help patients and families through difficult diagnoses as well as perform challenging, rewarding cases is what drew him to neurosurgery. His research interests include clinical vascular and spine related work. Away from medicine, Cole loves to spend his time with family and their lovely dog while also enjoying video games. He is also a huge history and music buff as he shares the title of workroom DJ with Austin and is good to have around for trivia nights. Cole loves the Roanoke nature and one of his favorite activities is exploring the greenway with his daughter and family when he’s not busy seeing patients.
Evan Courville, MD
Evan was born and raised in Lafayette, Louisiana. He attended Louisiana State University, earning degrees in mechanical engineering and biological sciences, and was a proud member of the LSU Tiger Band. Drawn by the intricate precision of both engineering and neurosurgery, he pursued medical school at LSU Health Sciences Center in New Orleans, graduating with research honors. Evan’s neurosurgery interests include technological advancements and TBI research, particularly the application of machine learning to enhance diagnostics and treatment. He aims to leverage his engineering background to improve patient care, especially in ICU settings where clinicians must continuously integrate vast amounts of data for real-time decision-making. Outside of work, Evan enjoys running, hiking, beach adventures, flying his drone and playing softball. His dedication to innovation and patient care drives his commitment to neurosurgery.
Abhishek “Abhi” Bhutada, MD
Abhi was born in Nagpur, India and grew up in Southern California. He attended undergrad at UC Berkeley earning a degree in molecular and cell biology. During his time in undergrad, he began working at a neuroimaging facility in UCSF as a research associate looking at various clinical applications for non-invasive imaging modalities, like Magnetoencephalography and functional-Magnetic Resonance Imaging. Working here sparked Abhi's interest in neurosurgery. Abhi went on to study medicine at Virginia Tech Carilion School of Medicine. He dedicated himself to many different research interests throughout medical school ranging from developing novel technologies to implementing advanced statistical modeling, graduating with an honors in research. Abhi is exploring all facets of neurosurgery but some of his neurosurgical interests include global neurosurgery and technological innovation. In his free time, Abhi enjoys hiking, playing basketball, long-distance running, playing Catan, attending music festivals and traveling.

Ousman Jallow, MD
Ousman Jallow has a multidisciplinary background spanning education, research, and industry. A native of The Gambia, he graduated as valedictorian of his high school class in 2012 before relocating to the United States to study Chemistry and Physics at the University of South Florida. His early career included teaching AP Chemistry and Physics and working as a research and development chemist in the pharmaceutical industry. Driven by a passion for medicine, he began his medical education at Meharry Medical College in 2019, graduating with high honors and induction into the Alpha Omega Alpha (AOA) Honor Medical Society. During medical school, he completed a dedicated year of neurosurgical research at Cedars-Sinai Medical Center. He went on to complete a preliminary year in general surgery at Carilion Clinic–Virginia Tech and is now excited to begin his neurosurgical training. Ousman has a strong interest in complex spinal procedures, pediatric neurosurgery, and global neurosurgery. Outside of medicine, he enjoys playing soccer, working out, running, and—most importantly—spending quality time with his son.
Our Collaborators
Many of our exciting research opportunities are made possible by the passion and partnership of our collaborators. We have had the pleasure to grow an extensive network of colleagues around the world who are passionate about understanding more about the nervous system and how those new findings can be used to give the best patient care possible.
Our partners include industry leaders in pharmaceuticals as well as academic and medical centers worldwide. Our research team continually looks for new ways to strengthen existing partnerships, foster new opportunities to further Carilion’s mission—and move medicine forward.
Industry Partners
Carilion Clinic partners with leading pharmaceutical companies and medical device manufacturers to research and develop new therapies to improve the quality of life for our patients. Technologies improve current treatments in pain, traumatic injury, movement disorder and paralysis have come to southwest Virginia through partnerships with companies such as:
- Boston Scientific
- DePuy Synthes
- Medtronic
- Mitsubishi
- Stryker
- Vivigen
____________
Academic Partners
Much of our success arises from our collaborations with outstanding researchers in academic research centers around the world.
Although we partner with many, we take advantage of the co-located Health Science Technology campus in Roanoke that incorporates Virginia Tech and Carilion Clinic. Below we highlight some of our collaborators from the Fralin Biomedical Research Institute at VTC and from Virginia Tech's main campus in Blacksburg, Virginia.

Center for Human Neuroscience
Fralin Biomedical Research Institute scientists in the Center for Human Neuroscience Research work with volunteer research participants from Roanoke, Blacksburg and the surrounding communities to understand decision-making, behavior and brain function and disorders in humans. Researchers in this Center have pioneered new approaches to human neuroimaging, including hyperscanning and magnetoencephalography using optically pumped magnetometry, as well as techniques to record and detect real-time fluctuations in neurochemicals while participants complete decision-making tasks.
____________
Chappell Lab
Dr. John Chappell received his bachelors in electrical engineering from the University of Virginia in 2001. Minoring in biomedical engineering and volunteering in the labs of Drs. Klaus Ley and Richard Price ignited his passion for biomedical research and blood vessels. He completed his Masters (2005) and Doctoral (2007) work in the lab of Dr. Richard Price at the University of Virginia, finding vascular biology and the microcirculation where he wanted to devote his career. This intrigue with the blood vasculature and its capacity to remodel led him to a postdoctoral fellowship in the lab of Dr. Victoria Bautch at the University of North Carolina at Chapel Hill, where he studied sprouting angiogenesis and the role of Flt-1 in regulating VEGF-A signaling (2007-2012). He pursued a second postdoctoral fellowship with support from an NIH K99/R00 Award (2012-2014), receiving additional training from Drs. Christer Betsholtz, Shayn Peirce-Cottler, and Feilim Mac Gabhann in pericyte biology and computational modeling of cellular and molecular interactions. He started his own lab in 2014 at the Fralin Biomedical Research Institute at Virginia Tech-Carilion (FBRI-VTC, Roanoke, VA) with a primary appointment in the Department of Biomedical Engineering and Mechanics at Virginia Tech (Blacksburg, VA). Dr. Chappell’s lab focuses primarily on microvascular pericytes -- their developmental origins and potential roles during vasculogenesis, their mechanistic involvement with sprouting angiogenesis, and their contributions to capillary stability and function in health and clinical conditions such as stroke and preeclampsia. Learn more about the Chappell Lab on Virginia Tech's website.
____________
Department of Engineering
Virginia Tech's Biomedical Engineering Department is a staunch supporter of community innovation and working with local healthcare professionals to help invent the future.
VISION: To be the premier program for improving the human condition and the communities we serve through our unique intersection of biomedical engineering, mechanics, and science.
MISSION: Our department leverages broad expertise in biomedical engineering, foundational mechanics, and sciences to solve significant health and technological challenges through cutting edge research, strategic partnerships, and educating students to be critical thinkers, and ethical innovators and leaders.
____________
Munson Lab
In the Munson Lab, we study the tumor microenvironment in cancers, including glioblastoma, the deadliest form of brain cancer. Our research focuses on the emerging research area of fluid flow. Cancer’s invasion of the brain follows distinctive routes that correlate with interstitial and bulk flow pathways. In brain cancer, fluid flow increases between cells within the tissue, specifically across the invasive edge of the tumor where cells are prone to both interact with the surrounding brain tissue and to evade localized, transport-limited therapies. We believe fluid flow can alter how a tumor responds to drug therapies. Not only is fluid flow important in cancer, but also is a contributor to normal function in tissues and other diseases. To this end, we are translating many of our methods and hypotheses to understand the role of fluid flow in immunity, aging, and Alzheimer’s Disease.
Our methods combine in vivo imaging methodology with in vitro tissue engineered models to examine the role of interstitial fluid flow and the cellular components of the microenvironment in cancer progression and treatment. We use patient-derived cells to create personalized models of disease to test hypotheses related to fluid and tissue transport in tumors and the brain and to identify new drug targets and treatment approaches.
Our goal is to find new targets in the tissue microenvironment by examining cancer and disease in the proper context, which includes dynamic fluid flows, multiple cell types, and patient-specific parameterization. By including the proper tissue environment, we believe that we can better identify underlying causes and contributors to disease and thus be better able to target and test new drugs for patients.
____________
Neural Dynamics Lab
Dr. Vijayan is the director of the Neural Dynamics and Neural Engineering (NDNE) Lab at Virginia Tech. The NDNE lab investigates the neural dynamics underlying diseased states, sleep-mediated memory consolidation, brain computer interface (BCI) applications, sensory processing, and mental imagery. The lab employs both invasive (intracranial) and non-invasive (EEG) techniques to examine neural activity, as well as signal processing and computational modeling techniques to make sense of the data they collect.
____________
Neurotrauma Research Lab
Michelle Theus is a Professor of Neurobiology in the Department of Biomedical Sciences, is the Co-Director of the Translational, Biology, Medicine and Health (TBMH) graduate program, Director of the VT-Neurotrauma Research Program and the Vice Chair for Precision Medicine at the Center for Engineered Health at Virginia Tech. She has received Virginia Tech’s Outstanding Mentor Award, Office of the Vice President for Research Scholar of the week and has been twice recognized by the NNS as a recipient of the Michael Goldberger Research Award and the Women in Neurotrauma Excellence Research Awards. She has held numerous council positions for the Central Virginia Chapter of the Society for Neuroscience and the National Capital Area for TBI Research. Her Laboratory of Neurotrauma & Repair is supported by the National Institute of Health and a multi-PI grant from the Epilepsy CURE foundation. Her work focuses on Eph receptor biology in brain injury with an emphasis on neurovascular and neuroimmune health.

____________
Pickrell Lab
The Pickrell Laboratory studies how mitochondrial dysfunction contributes to neurodegenerative diseases. We are particularly interested in how traumatic brain injury precipitates the risk of developing a neurodegenerative disease and how damaged mitochondria trigger innate immune responses in brain injury.

____________
The Schulz Group
Michael D. Schulz is an assistant professor in the Department of Chemistry at Virginia Tech, and a member of the Macromolecules Innovation Institute, the Center for Emerging, Zoonotic, and Arthropod-borne Pathogens, and the Virginia Tech Center for Drug Discovery. He received his Ph.D. in 2014 in organic and polymer chemistry and an M.S. in Pharmaceutical Science at the University of Florida under the supervision of Prof. Ken Wagener. After conducting research at the Max Planck Institute for Polymer Research as a Fulbright Scholar, he was a postdoctoral scholar in the group of Prof. Robert Grubbs at Caltech. He began his independent career at Virginia Tech in 2017. His diverse research interests span both fundamental and applied polymer chemistry, with a particular focus on materials in medicine. In collaboration with the Department of Neurosurgery, his research group is developing novel materials for neurosurgical applications. To learn more about The Schulz Group, click here.
____________
Global Initiatives
Carilion Neurosurgery has launched a new initiative to expand the neurosurgical capabilities to the public health care system of Nepal, which is home to 30 million people. The Carilion Neurosurgery department is currently working with two hospitals in Kathmandu, Nepal that provide excellent care to a very large population with diverse neurosurgical needs:
- Tribhuvan University of Nepal Institute of Medicine
- Bir Hospital
Our initiative focuses on research collaboration with multiple projects, such as the multinational study of Chiari malformation management from the two locations in the United States, Nepal and the Maldives. The Maldives have an exceptionally high incidence of Chiari malformation, which can lead to a multitude of issues arising from blocked flow of CSF and requiring surgery.

____________
Carilion Clinic Innovation
Carilion Clinic Innovation is a resource to develop, protect, market and appropriately capture value generated from employee-generated intellectual property (IP) and commercial innovations.
Conferences and Meetings
Congress of Neurological Surgeons
The Congress of Neurological Surgeons (CNS) is a leading organization dedicated to advancing neurosurgery through scientific discovery and its translation into clinical practice, education, and innovation. CNS has eight sections that operate independently but report to the executive board.
CNS holds annual meetings as well as education fellowships, online courses, webinars, podcasts and more.
American Association of Neurological Surgeons
The American Association of Neurological Surgeons (AANS) advances the specialty of neurological surgery through education, research, advocacy, and outcomes science to promote the highest quality of patient care. Our neurosurgeons publish, attend meetings as members of this esteemed body.
Society of Neurological Surgeons
The Society of Neurological Surgeons is the oldest neurosurgical professional society in the world. It was created by Harvey Cushing in 1920 and continues to focus on continuing development of the field of neurosurgery by including graduate and post-graduate education. They offer the Research Updates in Neuroscience for Neurosurgeons (RUNN) course, targeted to PGY3 and PGY4 residents, offering intensive exposure to some of the best neuroscience in the country.
Neurosurgical Society of the Virginias
The Organization of the Neurosurgical Society of Virginia began in 1965 and is the official state society for neurosurgeons in Virginia, West Virginia, and the surrounding mid-Atlantic region. Their objectives are to support continuing education in the field of neurosurgery among regional neurosurgeons and aligned professionals; provide a forum for the expression and dissemination of new scientific information among regional neurosurgeons and aligned professionals; have a recognized regional organization to speak for neurosurgical interests in social and economic affairs and promote a collegiate atmosphere for neurosurgeons in the Commonwealth and surrounding states.
Special Projects
Global Health

The Carilion Neurosurgery department is currently working with two highly regarded hospitals in Kathmandu, the largest and capital city of Nepal. Both Bir Hospital and Tribhuvan University of Nepal Institute of Medicine are considered public hospitals, as they derive the majority of their health care funding from government sources. They both have very well-respected training programs and provide excellent care to a very large population with diverse neurosurgical needs.
This global health initiative focuses on research collaboration with multiple projects ongoing (see Current Research), as well as collaborative international grand rounds and providing international surgical training to trainees from each program.
We are also making a concerted effort to expand the neurosurgical options available to these populations in the functional and endovascular realms of neurosurgery through direct and indirect surgical involvement. Our residents, fellows and attendings will rotate through these two hospitals, providing surgical care to Nepalese patients while teaching their counterparts techniques and skills utilized at Carilion Clinic. Our hosting partners will be sharing their expertise in specific diagnostic pathologies that have a high incidence rate not typically found in the southeastern U.S., as well as their unique health care resources and insights into patient care.
Together, this equal exchange of information, skills and patient care will be vital to improving both our understanding of unique pathologies as well as the skills needed to treat certain populations.
____________
Neurosurgery Repository
We have developed a central neural tissue, blood and cerebrospinal fluid (CSF) repository to be used in future research studies and/or educational training experiences at Carilion Clinic, Virginia Tech or other research institutions to study the underlying mechanisms of any neurosurgical involved disease or disorder.
Participants voluntarily consent before their surgery to donate the specimens (blood, tissue, CSF) that would already be collected but otherwise be discarded as waste. The goal of this bank is to build up a supply of tissues to be used in other studies looking to identify new treatment targets for neurosurgical-related conditions and improve patient outcomes.
Requesting Samples for Research
Researchers interested in using central nervous system tissue (brain or spinal), CSF or blood for a particular condition or control will need to submit a request to the Neural Tissue Repository Board for review, together with a project proposal detailing the protocol as well as the type and number of samples requested. The review board comprises neural specialists—scientists, researchers and clinicians—from Virginia Tech and Carilion Clinic.
Proposals approved by the board will then need to be submitted to the Carilion Clinic IRB with approval attached before specimens will be provided by the repository.
Getting Involved
Many students, patients, family members and members of the community often ask how they can become involved in research. The opportunities to support this vital work are as varied as the reasons behind their interests:
- Participating in research as a healthy volunteer to support research close to home
- Participating in research as patients to learn more about their own conditions and help develop potential treatments
- Offering scholarships or grant funding to further research endeavors
- Medical professionals who wish to experience research in the field for themselves
No matter what the method, we have a path for everyone to play a part in the exciting process of medical discovery.

Patient and Community Participation
Individuals in the community who are interested in participating in research have many opportunities available including assisting as healthy volunteers to compare baseline measures, to participating as a patient with specific medical conditions under study.
You can find more information about studies currently enrolling participants at the Current Research tab on this page and on Carilion Clinic's Research and Development page.
If you are interested in or have questions about participating in one of our Neurosurgery research projects at Carilion Clinic, please contact our director of Neurosurgery research:
Jordan Darden, Ph.D., CCRP
jadarden@carilionclinic.org
540-529-7792

____________
Clinical Research Internship
Advances in health care come from innovation and investigation of established and novel concepts. Observation of patients and the treatment success of trigger the burgeoning quest for improving patient lives and outcomes in as many ways as possible. Translating the observations from the clinic to a reductionist model to study the human condition and solve one more piece of the puzzle is the key part of translational science and taking that newfound knowledge and applying that back to help people is clinical research.
Clinical research encompasses not only the observation and investigation of human medical conditions, but also the use of research as a dynamic tool to help people. In order to be a useful tool, research must be clinically relevant, trusted and accepted by the population, and funded.
The Department of Neurosurgery is offering a clinical research internship to learn all aspects of clinical research, including:
- Ethics of human research
- Federal regulations and responsibilities of investigators
- Education and training for human research
- Institutional and business aspects of research
- Translational and clinical scientific skills
- Medical knowledge and academic discussion
- Funding for translational/clinical research
- Manuscripts preparation and submission
The clinical research intern is responsible for learning the basics of a clinical research trial. The goal of this program is to introduce the students to the concepts, lifecycle and execution of clinical trials and studies in medicine. The internship is an unpaid graduate level, semester-long opportunity for course credit only. The student will be required to work 30-40 hours per week.
Essential duties and responsibilities include:
- Working directly with a high-performing clinical research team in Carilion Clinic's department of Neurosurgery
- Helping design and develop a clinical development program, conduct literature searches, compile data and present reports
- Providing internal support to the clinical team by recruiting patients; collecting and analyzing data; and drafting manuscripts, grant proposals, project proposals and internal institutional applications and reports
- Liaising with laboratories and other study partners regarding research findings
- Participating as necessary, in cross-functional team meetings to ensure timely attainment of project milestones
- Working under the supervision and guidance of an experienced researcher
Due to the time and effort to onboard research interns, this internship is offered during the summer for a minimum of three months commitment. Onboarding will need to begin several months in advance of the time of anticipated start date.
Those interested may apply by completing the form below and emailing to jadarden@carilionclinic.org.
____________
Undergraduate Experience with Virginia Tech
NEUR 4594 Clinical Neuroscience in Practice (spring semesters)
This is a field study, experience-learning course for advanced Neuroscience students interested in careers in health care. The course combines highly interactive classroom meetings that include lectures, student-lead discussions, and paper presentations with experiential learning in the clinical theater of the Carilion Roanoke Memorial Hospital. Students will gain first-hand knowledge about currently available surgical treatment options for common neurological illnesses. They will witness patient care from diagnosis and pre-operative evaluation through surgery and post-surgical follow-up. Critical discussions throughout the course will include important issues and controversies in contemporary health care including, but not limited to, the role of technology in healthcare; increasing cost to society; health disparities and inequity in access to healthcare; patient versus societal responsibilities; decision-making in life-or-death situations; patient and doctor resilience; and burnout.
This course is a unique opportunity to learn from and shadow neurosurgeons in practice. As a student in this course, you will:
- learn the clinical approaches to diagnosing and treating neurological disorders and injuries faced by neurosurgeons;
- observe clinical procedures including diagnostic procedures, radiological techniques, and surgical procedures in the operating room;
- participate in physician-led didactic sessions and patient rounding;
- have opportunities to observe cadaver labs
This is not a typical class. Course work will include weekly summaries of what you learn during didactics and clinical observations, participation in class discussion, and weekly commitment to shadowing Carilion neurosurgeons. A significant amount of class time will be spent in clinical settings in Roanoke, so you will need to plan ample time in your schedule to attend these sessions and travel back and forth between Blacksburg and Roanoke.
Course Format and Meetings
The course has two major parts: weekly didactic lectures split between Roanoke and Blacksburg; and shadowing experiences in Carilion Roanoke Memorial Hospital.
Didactic Lectures in Blacksburg: certain Tuesdays, 9:30 - 10:45 a.m.
A subset of lectures will be given in Sandy Hall 012 on the Virginia Tech campus in Blacksburg; see calendar for dates.
Clinical Lectures in Roanoke - certain Tuesdays, 8 - 10 a.m.
A subset of Tuesday sessions will be spent at the Institute for Orthopedics and Neuroscience (ION) in Roanoke (2331 Franklin Rd SW, Roanoke, VA 24014, Conference Room 5; see calendar for dates). Students must plan to be present from 8-10 a.m. on these dates, and may remain until noon if their schedule allows. (Leaving Roanoke at 10 a.m. will allow sufficient time to return to Blacksburg for an 11 a.m. class.)
Cadaver Labs
One of the Tuesday morning Roanoke-based lectures will be held in the cadaver lab in the Virginia Tech Carilion School of Medicine (Riverside 2 Building, 2 Riverside Circle, Roanoke VA 24016, 3rd floor SPAL lab; see calendar for date). This session will run in two groups of 10 students, with each group spending 1 hour in the lab; more details will be provided as that session draws closer.
Clinical Shadowing in Roanoke – Wednesdays or Fridays, 8 a.m. - noon
The class will be divided into two ‘clinical groups’, with each assigned to observe surgeries at Carilion Roanoke Memorial Hospital (1906 Belleview Ave Roanoke, VA 24014) on Wednesday OR Friday mornings. Over the course of the semester, students will have a total of 12 shadowing sessions. Students & clinicians typically meet at 8:00 AM (Wed) and 7:00 AM (Fri) in the Higher Grounds coffee shop located on the ground floor of the hospital inside the Main Lobby. (Please note the differing start times on Wednesday vs. Friday; this reflects variation in Operating Room start times on these days.)This is a field study, experience-learning course for advanced Neuroscience students interested in careers in healthcare. The course combines highly interactive classroom meetings that include lectures, student-lead discussions, and paper presentations with experiential learning in the clinical theater of the Carilion Roanoke Memorial Hospital. Students will gain first-hand knowledge about currently available surgical treatment options for common neurological illnesses. They will witness patient care from diagnosis and pre-operative evaluation through surgery and post-surgical follow-up. Critical discussions throughout the course will include important issues and controversies in contemporary health care including, but not limited to, the role of technology in healthcare; increasing cost to society; health disparities and inequity in access to healthcare; patient versus societal responsibilities; decision-making in life-or-death situations; patient and doctor resilience; and burnout.
Students will learn to present patient cases and an actual case they have experienced in a “grand rounds” clinical presentation to the neurosurgery team. When students are preparing their end-of-semester patient case presentation, they will receive guidance from a clinical mentor. Students will be assigned mentors during the first two weeks of the semester.
Upon completion of this course, students should have gained factual knowledge; developed specific skills, competencies, and points of view needed by professionals in the field of Clinical Neuroscience and Neurological disease; and have developed skills in expressing oneself orally and in writing.
Time Requirements and other Prerequisites
Please note that the clinical excursion portion of the course requires you to commit to traveling to Carilion Roanoke Memorial Hospital (CRMH) once a week and spending an entire morning (Wednesdays OR Fridays 8:00am-12:00pm). Likewise, select Tuesday lecture sessions will be held in Roanoke at the Institute for Orthopedics and Neuroscience (ION) in Roanoke (2331 Franklin Rd SW, Roanoke, VA 24014).
Clinical Shadowing Requirements
To participate in the clinical shadowing, you must complete several Carilion Clinic requirements, including Health Insurance Portability and Accountability Act (HIPAA) training, up- to-date vaccinations, and evidence of a recent (within the last 12 months) tuberculosis (TB) test. Upon registering for the course, you will be contacted by Ms. Glenda Keller (glkeller@carilionclinic.org) from the Carilion Clinic Visiting Student Affairs Office. Ms. Keller will be your point of contact regarding these requirements, which must be completed to ensure participation in clinical shadowing as scheduled.
The course is designed for advanced undergraduates who have senior standing and have successfully completed: Introduction to Neuroscience I and II; Cellular and Molecular Neuroscience; and Diseases of the Nervous System (although this course can be taken concurrently). The number of students for the course this semester will be 20. If the course is oversubscribed, we will employ a fair, unbiased, and transparent selection process to determine student participants.

____________
Research Careers at Carilion
There are many opportunities to pursue a career in research as a part of the Carilion family. Carilion offers many careers in the research field, including clinical research coordinators and research assistants. Research assistants have a unique opportunity to gain experience for bachelors graduates before they pursue further education in graduate or medical school.
For more information about available job openings, please visit careers at Carilion Clinic.
FAQs and Resources
Frequently Asked Questions
Most patients, no matter their diagnosis, have a similar experience when it comes to visiting a neurosurgeon. There are differences in the diagnosis and treatment but some things such as timing of appointments remain consistent.
How can I contact my doctor?
If you have a general question and it is not an emergency, the one of the best ways to reach out to your care team is to send a message through MyChart. You can also call the ION office during business hours and speak to a nurse who will communicate with your doctor and call you back within 48 hours. If you have an emergency, call 911.
Does Carilion take my insurance?
Carilion Clinic contracts with most major health insurance carriers. This list is updated periodically and is subject to change without notice. Please contact your insurance company or health benefits manager to better understand how your policy works and any financial liability that you may have, prior to receiving care.
What happens if my insurance or circumstances change?
It’s very helpful if patients are made aware that if their insurance changes to notify the office as soon as possible, or surgery may have to be rescheduled. Especially, if prior authorization is needed.
I have to come quite a distance to see my doctor or to have my procedure. Where can I or my family stay during my medical care?
Carilion has specific hotels where patients can get a discount. Contact Guest Services at 540-981-7143 for information and to arrange your discount with the hotel.
How much will my procedure or surgery cost?
Our surgery schedules can provide the specific CPT code and diagnosis codes, which billing uses to calculate an estimated cost. There is more than one bill- the hospital charge, doctors charge, anesthesia charge which will come from Anesthesia Care of Virginia, and if any services such as Neuro monitoring is used, you will receive a bill from that as well.
Where can I get information about specific prices of my procedure or surgery?
You can contact our Billing Department at (540)983-4294 or toll free (866)720-3742. There is also a website that can provide an estimate of your potential costs. Pricing | Carilion Clinic
Surgery is very expensive. Is there any help I can get?
We provide financial assistance to eligible patients who receive emergency or other medically necessary care from us in any of our hospital facilities and/or from our clinicians. Financial assistance is only available for eligible services billed by Carilion Clinic. Please see our website for more information: Financial Assistance | Carilion Clinic
____________
Resources for Common Conditions
Receiving any neurological diagnosis can be scary. Most patients experience a whirlwind of emotions and thoughts when receiving any diagnosis from a specialist, especially from a neurosurgeon. Finding someone to support you and your family through this trying time is imperative as well as gathering as much reliable and accurate information as possible. We have gathered a list of resources common to several conditions to assist you in navigating these difficult times.
Listed below are some resources for brain cancer, Carilion support and care available for all patients.
News
News
Please check out our news stories and annual newsletter for the latest information about Neurosurgery Department endeavors.

Starting in 2024 Carilion Clinic Neurosurgery takes a moment to reflect on the year's journey. Click below to view our Annual Newsletters.




