COAPT Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients
Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation
About
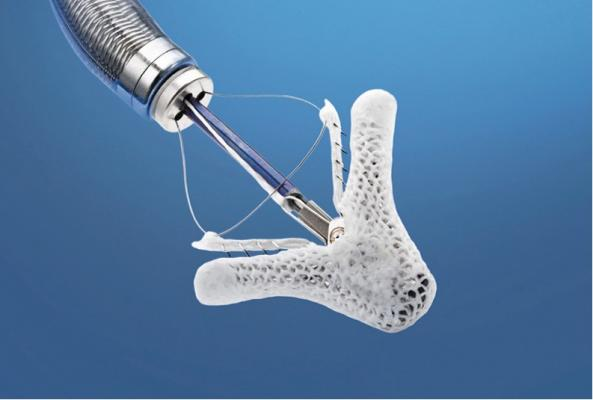
To evaluate the safety and effectiveness of the MitraClip System for the treatment of moderate-to-severe or severe functional mitral regurgitation (FMR) in symptomatic heart failure subjects who are treated per standard of care and who have been determined by the site’s local heart team as not appropriate for mitral valve surgery. This randomized controlled trial will provide the opportunity to strengthen or add labeling claims regarding safety and clinical benefits of the MitraClip System for symptomatic heart failure patients with moderate-to-severe or severe functional mitral regurgitation. Enrollment has closed.
Eligibility Criteria
Inclusion Criteria
- Subject has been adequately treated per applicable standards, including for coronary artery disease, left ventricular dysfunction, mitral regurgitation and heart failure
- Subject has had at least one hospitalization for heart failure in the 12 months prior to subject registration and/or a corrected BNP ≥300 pg/ml or corrected NT-proBNP ≥1500 pg/ml
- New York Heart Association (NYHA) Functional Class II, III or ambulatory IV
- Surgery will not be offered as a treatment option and medical therapy is the intended therapy for the subject
- Left ventricular ejection fraction (LVEF) is ≥20% and ≤50%
Exclusion Criteria
- Tricuspid valve disease requiring surgery
- Aortic valve disease requiring surgery
- Severe symptomatic carotid stenosis (>70% by ultrasound)
- Mitral valve orifice area <4.0 cm2
- Leaflet anatomy which may preclude MitraClip implantation, proper MitraClip positioning on the leaflets or sufficient reduction in MR by the MitraClip
Primary Investigator

Jason Foerst, M.D.
Medical director of the Structural Heart and Valve Center at Carilion Clinic and an assistant professor of Medicine at the Virginia Tech Carilion School of Medicine.